
Synthetic birnessites and buserites as heavy metal cation traps and environmental remedies
Kledi Xhaxhiu
The history of the name birnessite derives from the region called Birness, from where Jones and Milne reported at first on this material [1]. It importance is related to the its manganese content which classify it as the main Mn-containing phase in soil, marine and nodules [1, 2]. Beyond this fact, what makes this mineral important is its characteristic two dimensional layered structure built of edge- and corner-sharing MnO6 octahedral sheets separated by a single water molecule layer and random cations [4,5]. The latter cause a spacing varying from 7-7.1 Å [5]. Upon further hydrating at certain conditions they convert reversibly to busserites which have a similar structure but consist of two single molecule water layers enlarging the interlayer space around 10 Å [4,6,7]. Since water is loosely bound to the buserite structure, it can be lost easily upon drying yielding back birnessites [4,6,7]. This inter-conversion is of great importance for various topics and especially for manganese distribution in the nature. The special structure of birnessite and buserite bears several unusual properties such as pronounced adsorptive properties and ion exchanging [8-13]. In spite of the industrial applications of the latter [14-16, 17-20] deriving from this property, increasing interest is paid for their application as anticontamintats [10,12,21,22] in the environmental remediation. Numerous studies have unveiled their adsorbing/exchanging capacities of various alkaline metal cations [7,10,8,24], transition metals cations [9,11,13,22,23] or even hazardous nuclear wastes containing uranium cations [5]. Due to the importance of these materials and their continuously reevaluation, this study aims to shed light on the structural and adsorptive properties of Na-birnessite and emphasise their cation sorptive/exchanging properties
![Figure 1: Lecture by Associate Professor Vojtech
Adam of Mendel University in Brno on the role of
metallothionein in cancer [1]. houbova](pics/k1.jpg)
Figure 1: The reversible conversion of birnessite to dehydrated birnessite (left) and buserite (right). The top and bottom layers represent edge-sharing MnO6 octahedra. The given distances between octahedral layers are based on the literatures [4,5,7].
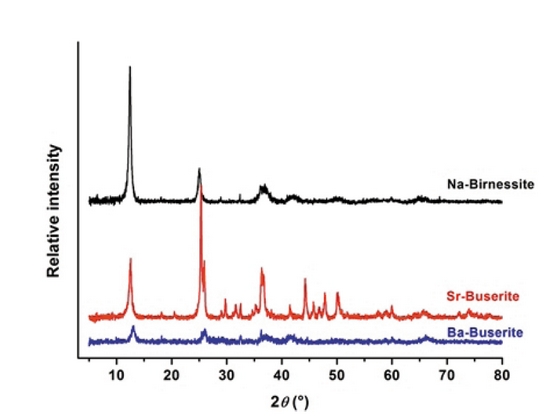
Figure 2: Measured XRD powder patterns of Na-birnessites, Sr- and Ba- busserites.
![Figure 1: Lecture by Associate Professor Vojtech
Adam of Mendel University in Brno on the role of
metallothionein in cancer [1]. houbova](pics/k3.jpg)
Figure 3: Adsorption desorption hysteresis of: (a) Na-birnessite, (c) Sr-buserite and (e) Ba-buserite; differential pore distribution of: (b) Na-birnessite, (d) Sr-buserite and (f) Ba-buserite.
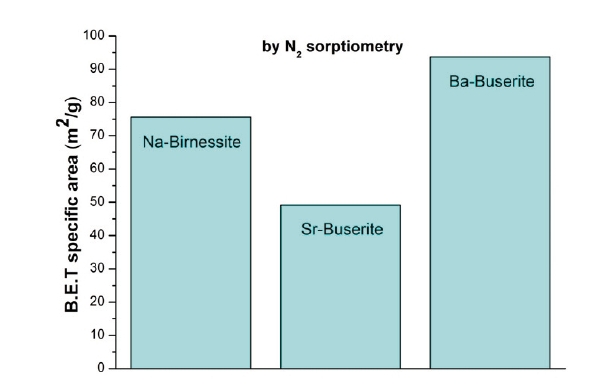
Figure 4: BET surface area of Na-birnessites, Sr- and Ba-buserites determined by N2 sorptiometry.
![Figure 1: Lecture by Associate Professor Vojtech
Adam of Mendel University in Brno on the role of
metallothionein in cancer [1]. houbova](pics/k5.jpg)
Figure 5: (a) Ar-adsorption isotherms of Na-birnessite and Ba-Buserite, (b) differential pore distribution of the samples of Na-birnessite and Ba-Buserite determined by Ar-sorptiometry.
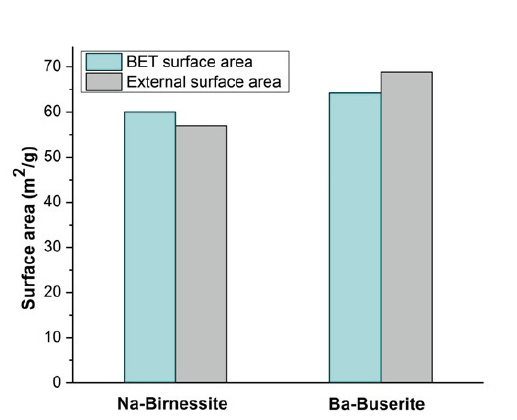
Figure 6: BET and external surface area of Na-birnessites, Sr- and Ba-buserites determined by Ar sorptiometry.
1. Jones, L. H. P., Milne, A. A. Mineral. Birnessite, a new manganese oxide mineral from Aberdeenshire, Scotland. Mineral. Mag., 1956, 31, 283-288.
2. Bricker, O. Some stability relations in system Mn-O2-H2O at 25 °C and 1 atmosphere total pressure. Am. Mineral., 1965, 50, 1296-1354.
3. Giovanoli, R.; Burki, P. Comparison of X-ray evidence of marine manganese nodules and non-marine manganese ore deposits. Chimia, 1975, 29, 266-269.
4. Le Goff, P., Baffler, N., Bach, S., Pereira-Ramos, J. P. Synthesis, ion exchange and electrochemical properties of lamellar phyllomanganates of the birnessite group. Mat. Res. Bul., 1996, 31(1), 63-75.
5. Al-Attar, L., Dyer, A. Sorption behaviour of uranium on birnessite, a layered manganese oxide. J. Mater. Chem., 2002, 12, 1381-1386.
6. Golden, D. C., Chen C. C., Dixon, J. B. Transformation of birnessite to buserite, todorokite, and manganite under mild hydrothermal treatment. Clays Clay Miner., 1987, 35(4), 271-280.
7. Luo, J., Zhang, Q., Huang, A., Giraldo, O., Suib, S. L. Double-Aging Method for Preparation of Stabilized Na-Buserite and Transformations to Todorokites Incorporated with Various Metals. Inorg. Chem., 1999, 38, 6106-6113.
8. Jeffries, D. S., Stumm, W. The metal-adsorption chemistry of buserite. Can.Mineral., 1976, 14, 16-22.
9. Mc Kenzie, R.M. The adsorption of lead and other heavy metals on oxides of manganese and iron. Aust. J. Soil Res., 1980, 18, 61-73.
10. Rives, V., Del Arco, M., Prieto, O. Birnesitas obtenidas mediante cambio iónico. Evolución estructural con la calcinación. (Birnessites obtained through ionic exchange. Structural evolution with the calcination). Bol. Soc. Esp. Ceram. V., 2004, 43(2), 142-147.
11. Feng, X. H., Zhai, L. M., Tan, W. F., Liu, F., He, J. Z. Adsorption and redox reactions of heavy metals on synthesized Mn oxide minerals. Environ Pollut., 2007, 147(2), 366-73.
12. Dias, A., Sá, R. G., Spitale, M. C., Athayde, M., Ciminelli, V. S.T. Microwave-hydrothermal synthesis of nanostructured Na-birnessites and phase transformation by arsenic(III) oxidation. Mat. Res. Bul., 2008, 43, 1528-1538.
13. Zhao, W., Wang, Q.Q., Liu, F. Pb2+ adsorption on birnessite affected by Zn2+ and Mn2+ pretreatments. J. Soils Sediment., 2010, 10, 870-878.
14. Pereira-Ramos, J. P., Badour, R., Bach, S., Baffier, N. Electrochemical and structural characteristics of some lithium intercalation materials synthesized via a sol-gel process: V2O5 and manganese dioxides-based compounds. Solid State Ionics, 1992, 701, 3-56.
15. Bach, S., Pereira-Ramos, J. P., Baffier, N. Electrochemical sodium insertion into the sol-gel birnessite manganese dioxide. Electrochim. Acta, 1993, 38, 1695-1700.
16. Bach, S., Pereira-Ramos, J. P., Baffier, N. Synthesis and Characterization of Lamellar MnO2 Obtained from Thermal Decomposition of NaMnO4 for Rechargeable Lithium Cells. J. Solid State Chem., 1995, 120, 70-73.
17. Shen, Y. F., Zerger, R. P., DeGuzman, R. N., Suib, S. L., McCurdy, L., Potter, D. I.; O’Young, C. L. Manganese oxide octahedral molecular sieves: preparation, characterization, and applications. J. Chem. Soc., Chem. Commun., 1992, 1213-1214.
18. Jiang, S. P., Ashton, W. R., Tseung, A. C. C. An observation of homogeneous and heterogeneous catalysis processes in the decomposition of H2O2 over MnO2 and Mn(OH)2. J. Catal., 1991, 131, 88-94.
19. Nitta, M. Characteristics of manganese nodules as adsorbents and catalysts, a review. Appl. Catal., 1984, 19, 151-176.
20. Yin, Y. G., Xu, W. Q., Shen, Y. F., Suib, L. S. Studies of Oxygen Species in Synthetic Todorokite-like Manganese Oxide Octahedral Molecular Sieves. Chem. Mater., 1994, 6(10), 1803-1808.
21. Wong, S.-T., Cheng, S. Synthesis and Characterization of Pillared Buserite. Inorg. Chem., 1992, 31, 1165-1172.
22. Gaillot, A.C., Drits, V.A., Manceau, A., Lanson, B. Structure of the synthetic K-rich phyllomanganate birnessite obtained by high-temperature decomposition of KMnO4: substructures of K-rich birnessite from 1000 °C experiment. Micropor. Mesopor. Mat., 2007, 98, 267-282.
23. Tebo, B.M., Bargar, J.R., Clement, B.G., Dick, G.J., Murray, K.J., Parker, D., Verity, R., Webb, S.M. Biogenic manganese oxides: properties and mechanisms of formation. Annu. Rev. Earth Planet. Sci., 2004, 32, 287-328.
24. Murray, D. J., Healey T. W., Fuerstenau, D. W. The adsorption of aqueous metal on colloidal hydrous manganese oxide. In Advances in Chemistry, Series 79, Adsorption from Aqueous Solution. Amer. Chem. Soc., 1968, 74-81.
25. Post, J. E., Veblen, D. R. Crystal-structure determinations of synthetic sodium, magnesium, and potassium birnessite using TEM and the Rietveld method. Am. Miner., 1990, 75, 477.
26. Prieto, O., Rives, V. Preparation and characterization of nonstoichiometric manganese oxides. Bol. Soc. Esp. Cerám. Vidrio, 2000, 39(3), 233-238.
27. Brock, S. L., Duan, N., Tian, Z. R., Giraldo, O., Zhou H., Suib. S. L. A review of porous manganese oxide materials. Chem. Mater., 1998, 10, 2619-2628.
28. K. Kuma, A. Usui, W. Palawsky, B. Gedulin y G. Arrhenius. “Crystal structure of synthetic 7 Å and 10 Å manganates substituted by mono- and divalent cations”. Miner. Mag., 1994, 58, 425-447.
29. Breck, D. W. Zeolite Molecular Sieves; Wiley: New York, 1973, pp 636.
30. Cornell, R. M., Giovanoli, R. Transformation of hausmannite into birnessite in alkaline media. Clays Clay Miner., 1988, 36(3), 249-257.
31. Shannon, R. D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Cryst., 1976, A32, 751-767.
32. Villalobos, M., Bargar, J., Sposito, G., Mechanisms of Pb(II) sorption on a biogenic manganese oxide. Environ. Sci. & Technol., 2005, 39, 569-576.
J.Met.Nano:
volume-2, issue-3
- Laboratory of Metallomics and Nanotechnologies – an initiator of the Metallomics Scientific Network formation
- Capillary electrophoresis of metallothionein
- Synthetic birnessites and buserites as heavy metal cation traps and environmental remedies
- Immunohistochemical detection of metallothionein
- MALDI-TOF MSI and electrochemical detection of metallothionein in chicken liver after cadmium exposure
- The use of MALDI MSI for the study of different tissues
- Utilization of graphene oxide electrophoretic deposition for construction of electrochemical sensors and biosensors
- Influence of Different Inducers on Ligninolytic Enzyme Activities
- Interaction of nanocarrier apoferritin with cytotoxic drug molecules
- Study of cell penetrating peptide and Europium(III) and Terbium(III) Schiff base complexes interaction
- HPV Detection in Leukocyte Samples of Spinocellular Carcinomas Using PCR
- Characterization of carbon quantum dots by capillary electrophoresis with laser-induced fluorescence detections
- Modification of anti-DNA antibodies with carbon quantum dots
- Fluorescence detection of carbon quantum dots assessed by stratospheric platform
 PDF
PDF