
Study of cell penetrating peptide and Europium(III) and Terbium(III) Schiff base complexes interaction
Vedran Milosavljevic, Pavel Kopel, Miguel Angel Merlos Rodrigo , Kristyna Cihalova, Amitava Moulick, Dorota Wawrzak and Rene Kizek
During the last decade newly developed potential therapeutic drugs such as proteins, nucleic acids, and new types of hydrophilic drugs are being reported. However, these drugs mostly have limitations due to their inability to reach the appropriate intracellular targets as a consequence of poor possibility to penetrate through cell membranes or deactivation by resistance mechanisms that transport these compounds out of the cell, both limiting their interaction with intracellular targets [1,2]. It has been also reported that poor cell specificity and normal cell cytotoxicity are common in application of standard technique for drug delivery systems, such as microinjection, electroporation, liposomal formulation and use of viral vectors [3-5]. In the search for new anticancer agents and drug delivery systems, cell penetrating peptides (CPP) attracted attention due to their possibilities for intracellular delivery of a wide range of macromolecules. CPP are short peptides consisting of less than 30 amino acids. CPP structures are mostly composed of positively charged amino acids (e.g. Arg, Lys and His) providing them possibility to translocate through the cell membrane by various mechanism, including endocytosis, and easily deliver various cell-impermeable covalently or noncovalently conjugated bioactive cargo such as proteins [6], nucleic acid [7], siRNA [8], peptide nucleic acid [9] and quantum dots [10]. CPPs as delivery agents were in focus of many investigations with the aim to increase stability and efficiency of cargo delivery avoiding the problem of cytotoxicity effect, lack of cell specificity and unexpected side effects. However, it has been shown that side effects on normal cells during cancer therapy or antibacterial application are minimized [11-13]. Peptides as drug carriers offer some advantages over other carriers as they are relatively easy to modify with various organic or inorganic materials, especially with compounds that have fluorescent properties, enabling easy tracking of drugs after application and for better understanding of the structure and functions of biological systems [14]. Many of luminescent materials such as organic fluorophores [15], recombinant proteins [16], semiconductor nanoparticles [17], and emissive metal complexes [18] are being used for peptide labeling. Application of organic dyes has many limitations associated with poor extinction coefficient or quantum yield and low stability against bleaching. However, on the other hand, metal complexes, especially rare earth metal-based materials show excellent optical properties since the f−f emission lines of Pr(III), Sm(III), Eu(III), Tb(III), Dy(III) and Tm(III) ions are in the visible range [19,20]. Especially great attention attracts luminescent rare-earth metal complexes with Eu(III) and Tb(III) as the excited states of these ions are less sensitive to vibrational quenching by intra or intermolecular energy transfer to adjacent high-energy vibrators such as hydroxyl groups [21]. Comparing metal-rare complex with quantum dots (QDs), they have long life time fluorescence and the fluorescence wavelength of Ln(III) ions is not sensitive to particle size, where the study of the function and properties of these compounds is simplified in comparison to QDs [22]. However, Ln(III) complexes are mostly used for study of their magnetic properties and imagine purpose, only few scientific reports are dealing with application of the complexes in biological applications [23-25]. Based on this consideration, we were interested in preparation of Schiff bases 2-[(E)-2-pyridylmethyleneamino]-N-[2-[(E)-2-pyridylmethyleneamino]ethyl]ethanamine (S-5) and 2-[(E)-2-pyridylmethyleneamino]-N,N-bis[2-[(E)-2-pyridylmethyleneamino]ethyl]ethanamine (S-6) and their europium(III) and terbium(III) complexes Eu(III)-S-5 and Tb(III)-S-6 with luminescent properties in order to study their interaction with cell penetrating peptide and possible biological applications. The compounds were evaluated against several bacterial species with respect to their toxicity.
![Figure 1: Lecture by Associate Professor Vojtech
Adam of Mendel University in Brno on the role of
metallothionein in cancer [1]. houbova](pics/vedran-1.jpg)
Figure 1: (a) Absorption and fluorescence spectra of cell penetrating peptide, Tb(III)-S-5 and Tb(III)-S-6 Schiff base complex. (b) Absorption and fluorescence spectra of cell penetrating peptide, Eu(III)-S-5 and Eu(III)-S-6 Schiff base complex.
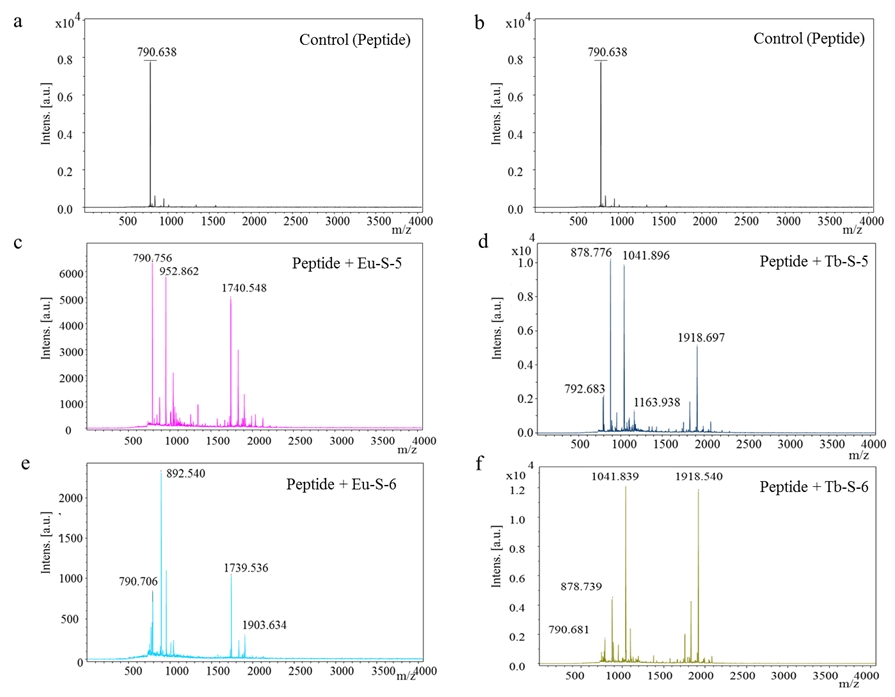
Figure 2: (a,b) Mass spectra of peptide. (c) Mass spectrum of peptide interaction with Eu(III)-S-5 complex, (d) Tb(III)-S-5 complex, (e) Eu(III)-S-6 complex, (f) Tb(III)-S-6 complex.
![Figure 1: Lecture by Associate Professor Vojtech
Adam of Mendel University in Brno on the role of
metallothionein in cancer [1]. houbova](pics/vedran-3.jpg)
Figure 3: Spectrophotometric determination of growth curves obtained by treatment with 0, 0.125, 0.312, 0.625, 1, 2.5 and 5 mM concentration of Eu(III) and Tb(III) Schiff base complex conjugate with cell penetrating peptide of: (a) E. coli after application of Eu(III)-S-5 conjugate with CPP. (b) E. coli after application of Eu(III)-S-6 conjugate with CPP. (c) E. coli after application of Tb(III)-S-5 conjugate with CPP. (d) E. coli after application of Tb(III)-S-6 conjugate with CPP.
1. Torchilin, V. Intracellular delivery of protein and peptide therapeutics. Drug discovery today. Technologies 2008, 5, e95-e103.
2. Perez-Tomas, R. Multidrug resistance: Retrospect and prospects in anti-cancer drug treatment. Current Medicinal Chemistry 2006, 13, 1859-1876.
3. Pujals, S.; Giralt, E. Proline-rich, amphipathic cell-penetrating peptides. Advanced Drug Delivery Reviews 2008, 60, 473-484.
4. Elmquist, A.; Langel, U. In vitro uptake and stability study of pvec and its all-d analog. Biological Chemistry 2003, 384, 387-393.
5. Vives, E.; Brodin, P.; Lebleu, B. A truncated hiv-1 tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. Journal of Biological Chemistry 1997, 272, 16010-16017.
6. Floren, A.; Maeger, I.; Langel, U. Uptake kinetics of cell-penetrating peptides. In Cell-penetrating peptides: Methods and protocols, Langel, U., Ed. 2011; Vol. 683, pp 117-128.
7. Johnson, L.N.; Cashman, S.M.; Kumar-Singh, R. Cell-penetrating peptide for enhanced delivery of nucleic acids and drugs to ocular tissues including retina and cornea. Molecular Therapy 2008, 16, 107-114.
8. Chiu, Y.L.; Ali, A.; Chu, C.Y.; Cao, H.; Rana, T.M. Visualizing a correlation between sirna localization, cellular uptake, and rnai in living cells. Chemistry & Biology 2004, 11, 1165-1175.
9. Turner, J.J.; Ivanova, G.D.; Verbeure, B.; Williams, D.; Arzumanov, A.A.; Abes, S.; Lebleu, B.; Gait, M.J. Cell-penetrating peptide conjugates of peptide nucleic acids (pna) as inhibitors of hiv-1 tat-dependent trans-activation in cells. Nucleic Acids Research 2005, 33, 6837-6849.
10. Yukawa, H.; Kagami, Y.; Watanabe, M.; Oishi, K.; Miyamoto, Y.; Okamoto, Y.; Tokeshi, M.; Kaji, N.; Noguchi, H.; Ono, K., et al. Quantum dots labeling using octa-arginine peptides for imaging of adipose tissue-derived stem cells. Biomaterials 2010, 31, 4094-4103.
11. Heitz, F.; Morris, M.C.; Divita, G. Twenty years of cell-penetrating peptides: From molecular mechanisms to therapeutics. British Journal of Pharmacology 2009, 157, 195-206.
12. Geisler, I.; Chmielewski, J. Cationic amphiphilic polyproline helices: Side-chain variations and cell-specific internalization. Chemical Biology & Drug Design 2009, 73, 39-45.
13. Mussbach, F.; Franke, M.; Zoch, A.; Schaefer, B.; Reissmann, S. Transduction of peptides and proteins into live cells by cell penetrating peptides. Journal of Cellular Biochemistry 2011, 112, 3824-3833.
14. Miller, L.W.; Cornish, V.W. Selective chemical labeling of proteins in living cells. Current Opinion in Chemical Biology 2005, 9, 56-61.
15. Domaille, D.W.; Que, E.L.; Chang, C.J. Synthetic fluorescent sensors for studying the cell biology of metals. Nature Chemical Biology 2008, 4, 168-175.
16. Shaner, N.C.; Steinbach, P.A.; Tsien, R.Y. A guide to choosing fluorescent proteins. Nature Methods 2005, 2, 905-909.
17. Weng, J.F.; Ren, J.C. Luminescent quantum dots: A very attractive and promising tool in biomedicine. Current Medicinal Chemistry 2006, 13, 897-909.
18. Gonzalez, D.; Lokhande, N.; Vadde, S.; Zhao, Q.; Cassill, A.; Renthal, R. Luminescence resonance energy transfer in the cytoplasm of live escherichia coli cells. Biochemistry 2011, 50, 6789-6796.
19. Eliseeva, S.V.; Buenzli, J.-C.G. Lanthanide luminescence for functional materials and bio-sciences. Chemical Society Reviews 2010, 39, 189-227.
20. Bunzli, J.C.G.; Piguet, C. Taking advantage of luminescent lanthanide ions. Chemical Society Reviews 2005, 34, 1048-1077.
21. Dickins, R.S.; Parker, D. Signalling reversible anion binding in aqueous media. Macrocyclic Chemistry: Current Trends and Future Perspectives 2005, 121-+.
22. Shen, J.; Sun, L.-D.; Yan, C.-H. Luminescent rare earth nanomaterials for bioprobe applications. Dalton Transactions 2008, 5687-5697.
23. Zangana, K.H.; Pineda, E.M.; Winpenny, R.E.P. Tetrametallic lanthanide(iii) phosphonate cages: Synthetic, structural and magnetic studies. Dalton Transactions 2014, 43, 17101-17107.
24. Wu, Y.; Morton, S.; Kong, X.; Nichol, G.S.; Zheng, Z. Hydrolytic synthesis and structural characterization of lanthanide-acetylacetonato/hydroxo cluster complexes - a systematic study. Dalton Transactions 2011, 40, 1041-1046.
25. Hauser, C.P.; Thielemann, D.T.; Adlung, M.; Wickleder, C.; Roesky, P.W.; Weiss, C.K.; Landfester, K. Luminescent polymeric dispersions and films based on oligonuclear lanthanide clusters. Macromolecular Chemistry and Physics 2011, 212, 286-296.
26. Thielemann, D.T.; Wagner, A.T.; Roesch, E.; Koelmel, D.K.; Heck, J.G.; Rudat, B.; Neumaier, M.; Feldmann, C.; Schepers, U.; Braese, S., et al. Luminescent cell-penetrating pentadecanuclear lanthanide clusters. Journal of the American Chemical Society 2013, 135, 7454-7457.
27. Galindo-Murillo, R.; Cheatham, T.E., III. DNA binding dynamics and energetics of cobalt, nickel, and copper metallopeptides. Chemmedchem 2014, 9, 1252-1259.
28. Shintoyo, S.; Fujinami, T.; Matsumoto, N.; Tsuchimoto, M.; Weselski, M.; Bienko, A.; Mrozinski, J. Synthesis, crystal structure, luminescent and magnetic properties of europium and terbium complexes with a bidentate benzoate and a tripod n-7 ligand containing three imidazole, ln(iii)(h3l)benzoate (clo4)(2)center dot h2o center dot 2meoh (ln(iii) = eu-iii and tb-iii). Polyhedron 2015, 91, 28-34.
29. Bassett, A.P.; Magennis, S.W.; Glover, P.B.; Lewis, D.J.; Spencer, N.; Parsons, S.; Williams, R.M.; De Cola, L.; Pikramenou, Z. Highly luminescent, triple- and quadruple-stranded, dinuclear eu, nd, and sm(iii) lanthanide complexes based on bis-diketonate ligands. Journal of the American Chemical Society 2004, 126, 9413-9424.
30. Hampe, O.; Klyatskaya, S.; Karpuschkin, T.; Vonderach, M.; Weis, P.; Ruben, M.; Kappes, M.M. Mass spectrometric characterization of a dinuclear terbium phthalocyaninato complex. International Journal of Mass Spectrometry 2012, 325, 183-188.
31. Martinez-Abad, A.; Sanchez, G.; Lagaron, J.M.; Ocio, M.J. On the different growth conditions affecting silver antimicrobial efficacy on listeria monocytogenes and salmonella enterica. International Journal of Food Microbiology 2012, 158, 147-154.
32. Percival, S.L.; Thomas, J.; Linton, S.; Okel, T.; Corum, L.; Slone, W. The antimicrobial efficacy of silver on antibiotic-resistant bacteria isolated from burn wounds. International Wound Journal 2012, 9, 488-493.
33. Chohan, Z.H.; Farooq, M.A.; Scozzafava, A.; Supuran, C.T. Antibacterial schiff bases of oxalyl-hydrazine/diamide incorporating pyrrolyl and salicylyl moieties and of their zinc(ii) complexes. Journal of Enzyme Inhibition and Medicinal Chemistry 2002, 17, 1-7.
34. Agh-Atabay, N.M.; Dulger, B.; Gucin, F. Structural characterization and antimicrobial activity of 1,3-bis(2-benzimidazyl)-2-thiapropane ligand and its pd(ii) and zn(ii) halide complexes. European Journal of Medicinal Chemistry 2005, 40, 1096-1102.
35. Osowole, A.A.; Kolawole, G.A.; Fagade, O.E. Synthesis, characterization and biological studies on unsymmetrical schiff-base complexes of nickel(ii), copper(ii) and zinc(ii) and adducts with 2,2 ‚-dipyridine and 1,10-phenanthroline. Journal of Coordination Chemistry 2008, 61, 1046-1055.
J.Met.Nano:
volume-2, issue-3
- Laboratory of Metallomics and Nanotechnologies – an initiator of the Metallomics Scientific Network formation
- Capillary electrophoresis of metallothionein
- Synthetic birnessites and buserites as heavy metal cation traps and environmental remedies
- Immunohistochemical detection of metallothionein
- MALDI-TOF MSI and electrochemical detection of metallothionein in chicken liver after cadmium exposure
- The use of MALDI MSI for the study of different tissues
- Utilization of graphene oxide electrophoretic deposition for construction of electrochemical sensors and biosensors
- Influence of Different Inducers on Ligninolytic Enzyme Activities
- Interaction of nanocarrier apoferritin with cytotoxic drug molecules
- Study of cell penetrating peptide and Europium(III) and Terbium(III) Schiff base complexes interaction
- HPV Detection in Leukocyte Samples of Spinocellular Carcinomas Using PCR
- Characterization of carbon quantum dots by capillary electrophoresis with laser-induced fluorescence detections
- Modification of anti-DNA antibodies with carbon quantum dots
- Fluorescence detection of carbon quantum dots assessed by stratospheric platform
 PDF
PDF