
Characterization of carbon quantum dots by capillary electrophoresis with laser-induced fluorescence detections
Marketa Vaculovicova, Simona Dostalova, Vedran Milosavljevic, Pavel Kopel, Vojtech Adam and Rene Kizek
Recently, semiconductor quantum dots (QDs) have been established as a valuable tool for labeling and sensing [1-3]. However, semiconductor quantum dots possess certain limitations such as high toxicity due to the use of heavy metals in their production. It is known that heavy metals are highly toxic even at relatively low levels, which may prove prohibitive to any clinical studies. This prompted the creation of carbon-based fluorescent nanoparticles (CQDs) to replace semiconductor QDs due to their low toxicity, biocompatibility, low cost and chemical inertness in addition to having similar fluorescence properties [4]. Similarly to semiconductor QD, fluorescent carbon nanoparticles can employed for chemical sensing applications - monitoring of metal ion content [5,6], pH sensing [7], biosensing [8] and/or in vivo imaging [9,10].
Even though the capillary electrophoresis (CE) is an extremely valuable separation analytical method and in combination with laser-induced fluorescence detection provides exceptionally low limits of detection, its application to analysis of fluorescent carbon nanomaterials is relatively limited [11]. In contrast to stationary fluorescence spectrometry, CE is capable to reveal the presence of various species in the sample due to their different electrophoretic mobility.
In this work, preparation of CQDs from various precursors such as citric acid, sucrose and multiwall carbon nanotubes were synthetized, optically characterized by fluorescence spectrometry and investigated by capillary electrophoresis with laser-induced fluorescence detection.
![Figure 1: Lecture by Associate Professor Vojtech
Adam of Mendel University in Brno on the role of
metallothionein in cancer [1]. houbova](pics/vac1.jpg)
Figure 1: Photographs of various types of CQDs solutions (MWCNT-CQDs, S-CQDs, PEG-CA- -CQDs) under UV (254 and 312 nm) and ambient light illumination.
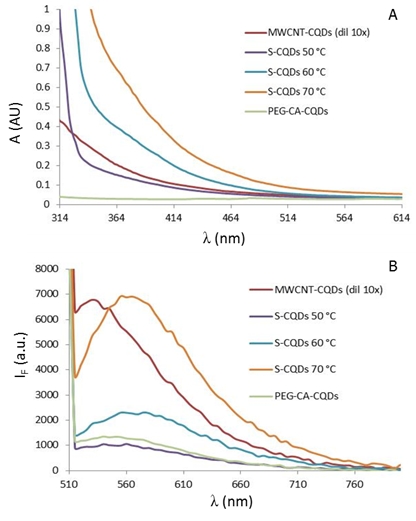
Figure 2: (A) Absorbance spectra of synthetized CQDs. (B) Emission spectra of synthetizes CQDs after excitation by 480 nm.
![Figure 1: Lecture by Associate Professor Vojtech
Adam of Mendel University in Brno on the role of
metallothionein in cancer [1]. houbova](pics/vac3.jpg)
Figure 3: CE-LIF of PEG-CA-CQDs. Separation conditions: internal diameter - 75 µm, length - 54/64.5 cm, separation voltage - 20 kV, hydrodynamic injection - 0.5 psi, 5 s, electrolyte - 20 mM sodium borate, pH 9.
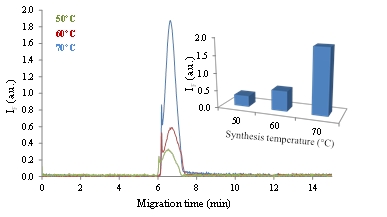
Figure 4: CE-LIF of S-CQDs prepared by using 50, 60 and 70 °C. Separation conditions: internal diameter - 75 µm, length - 54/64.5 cm, separation voltage - 20 kV, hydrodynamic injection - 0.5 psi, 5 s, electrolyte - 20 mM sodium borate, pH 9. Inset: Dependence of the peak height on the preparation temperature.
![Figure 1: Lecture by Associate Professor Vojtech
Adam of Mendel University in Brno on the role of
metallothionein in cancer [1]. houbova](pics/vac5.jpg)
Figure 5: CE-LIF of MWCNT-CQDs. Separation conditions: internal diameter - 75 µm, length - 54/64.5 cm, separation voltage - 20 kV, hydrodynamic injection - 0.5 psi, 5 s, electrolyte - 20 mM sodium borate, pH 9. Inset: Dependence of the peak height on the dilution by separation electrolyte.
1. Chan, W.C.W.; Maxwell, D.J.; Gao, X.H.; Bailey, R.E.; Han, M.Y.; Nie, S.M. Luminescent quantum dots for multiplexed biological detection and imaging. Curr. Opin. Biotechnol. 2002, 13, 40-46.
2. Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater. 2005, 4, 435-446.
3. Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307, 538-544.
4. Lim, S.Y.; Shen, W.; Gao, Z.Q. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362-381.
5. Guo, Y.M.; Zhang, L.F.; Zhang, S.S.; Yang, Y.; Chen, X.H.; Zhang, M.C. Fluorescent carbon nanoparticles for the fluorescent detection of metal ions. Biosens. Bioelectron. 2015, 63, 61-71.
6. Yin, P.P.; Ai, K.L.; Li, M.L.; Sun, G.Y. Highly fluorescent carbon nanoparticles for sensitive detection of iron (iii). Chin. J. Anal. Chem. 2014, 42, 1427-1433.
7. Li, H.T.; Ming, H.; Liu, Y.; Yu, H.; He, X.D.; Huang, H.; Pan, K.M.; Kang, Z.H.; Lee, S.T. Fluorescent carbon nanoparticles: Electrochemical synthesis and their ph sensitive photoluminescence properties. New J. Chem. 2011, 35, 2666-2670.
8. Ouyang, X.Y.; Liu, J.H.; Li, J.S.; Yang, R.H. A carbon nanoparticle-based low-background biosensing platform for sensitive and label-free fluorescent assay of DNA methylation. Chem. Commun. 2012, 48, 88-90.
9. Fang, Y.X.; Guo, S.J.; Li, D.; Zhu, C.Z.; Ren, W.; Dong, S.J.; Wang, E.K. Easy synthesis and imaging applications of cross-linked green fluorescent hollow carbon nanoparticles. ACS Nano 2012, 6, 400-409.
10. Ruan, S.B.; Wan, J.Y.; Fu, Y.; Han, K.; Li, X.; Chen, J.T.; Zhang, Q.Y.; Shen, S.; He, Q.; Gao, H.L. Pegylated fluorescent carbon nanoparticles for noninvasive heart imaging. Bioconjugate Chem. 2014, 25, 1061-1068.
11. Hu, Q.; Paau, M.C.; Zhang, Y.; Chan, W.; Gong, X.J.; Zhang, L.; Choi, M.M.F. Capillary electrophoretic study of amine/carboxylic acid-functionalized carbon nanodots. J. Chromatogr. A 2013, 1304, 234-240.
J.Met.Nano:
volume-2, issue-3
- Laboratory of Metallomics and Nanotechnologies – an initiator of the Metallomics Scientific Network formation
- Capillary electrophoresis of metallothionein
- Synthetic birnessites and buserites as heavy metal cation traps and environmental remedies
- Immunohistochemical detection of metallothionein
- MALDI-TOF MSI and electrochemical detection of metallothionein in chicken liver after cadmium exposure
- The use of MALDI MSI for the study of different tissues
- Utilization of graphene oxide electrophoretic deposition for construction of electrochemical sensors and biosensors
- Influence of Different Inducers on Ligninolytic Enzyme Activities
- Interaction of nanocarrier apoferritin with cytotoxic drug molecules
- Study of cell penetrating peptide and Europium(III) and Terbium(III) Schiff base complexes interaction
- HPV Detection in Leukocyte Samples of Spinocellular Carcinomas Using PCR
- Characterization of carbon quantum dots by capillary electrophoresis with laser-induced fluorescence detections
- Modification of anti-DNA antibodies with carbon quantum dots
- Fluorescence detection of carbon quantum dots assessed by stratospheric platform
 PDF
PDF