
Modification of anti-DNA antibodies with carbon quantum dots
Simona Dostalova, Marketa Vaculovicova, Sona Krizkova, Lukas Richtera, Pavel Kopel and Rene Kizek
Antibodies represent a specialized glycoprotein group and form the main base of vertebrate immune system. There are 5 immunoglobulin classes in mammalian serum: IgG, IgM, IgA, IgD and IgE [1]. In avian species the immunoglobulins G are replaced by highly functionally similar immunoglobulins Y and there are also IgA and IgM present [2]. Antibodies are often used in variety of applications, including ELISA for the detection of various analytes [3], virions [4] or tumor markers [5], cell capture for subsequent analysis [6] and magnetic immunoseparation [7,8]. For bulk production, antibodies extracted from chicken egg yolks immunized with the specific antigen are often used [9].
Anti-DNA antibodies were first discovered in 1957 [10] and they can bind to single stranded or double stranded DNA [11,12]. Anti-dsDNA antibodies can be found in the blood of systemic lupus erythematodes (SLE) patients. This autoimmune disease is manifesting by formation of immunocomplexes [13]. The antibodies usually belong to class IgM in normal patients or IgG in SLE patients [14] and they can be transiently found in the blood of patients with some viral infections (HIV, BK or B19 virus) [15]. Anti-dsDNA antibodies usually bind to sugar-phosphate backbone, base pairs or some double strand conformations. Anti-ssDNA antibodies should be able to bind to bases, nucleotides, oligonucleotides and sugar-phospate backbone [16]. Commercially available are also antibodies against DNA adducts such as those with cisplatin [17]. The combination of suitable antibodies can help identify the secondary structures of DNA [18].
To enhance the antigen detection limit it is possible to label the antibodies with highly fluorescent nanoparticles such as quantum dots (QDs) [3]. QDs have unique fluorescent characteristics, including wide absorbance spectrum with narrow emission spectrum, high quantum yield or excellent photostability [19]. They are usually formed by a semiconductor crystals, such as CdTe, CdS or ZnS, but they can also be prepared from carbon materials with polymer coating [6]. These materials include nanodiamonds [20], graphene [21], graphite [22], single- and multi-wall carbon nanotubes [23], citric acid [24] or sucrose [25]. The prepared carbon quantum dots usually show high fluorescence under UV light [26].
In this work, we studied anti-DNA antibodies produced in chicken egg yolks as a tool for DNA structure detection. We used four different anti-DNA antibodies: anti-dsDNA, afi-dsDNA, anti-ssDNA and afi-ssDNA. Their reactivity towards various sized DNA molecules was evaluated using dot blot method. The successful modification of anti-DNA antibodies with carbon quantum dots was determined by ELISA-like method with fluorescent detection. .
![Figure 1: Lecture by Associate Professor Vojtech
Adam of Mendel University in Brno on the role of
metallothionein in cancer [1]. houbova](pics/dostalova-1.jpg)
Figure 1: The dot blot assay for evaluation of antibody reactivity with single and double stranded DNA of different length. (A) The sample order on Zeta Probe membrane. (B) Dot blot assay with 15 nM DNA and 22 μg/mL of antibodies. (C) Dot blot assay with 100 μg/mL of DNA and 22 μg/mL of antibodies.
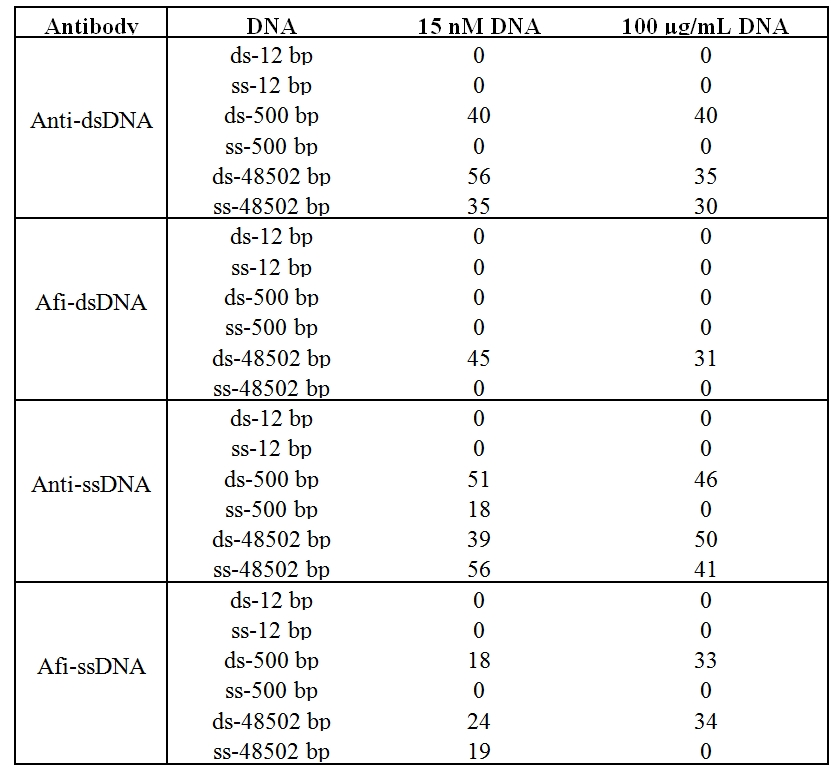
Table 1: The colorimetric intensity quantification of dot blot assay.
![Figure 1: Lecture by Associate Professor Vojtech
Adam of Mendel University in Brno on the role of
metallothionein in cancer [1]. houbova](pics/dostalova3.jpg)
Figure 2: The characterization of MWCNT-CQDs. (A) Visualization under ambient and UV (254 and 312 nm) light. (B) Absorbance spectrum of MWCNT-CQDs. (C) The fluorescence spectra of MWCNT-CQDs after various excitation wavelength.
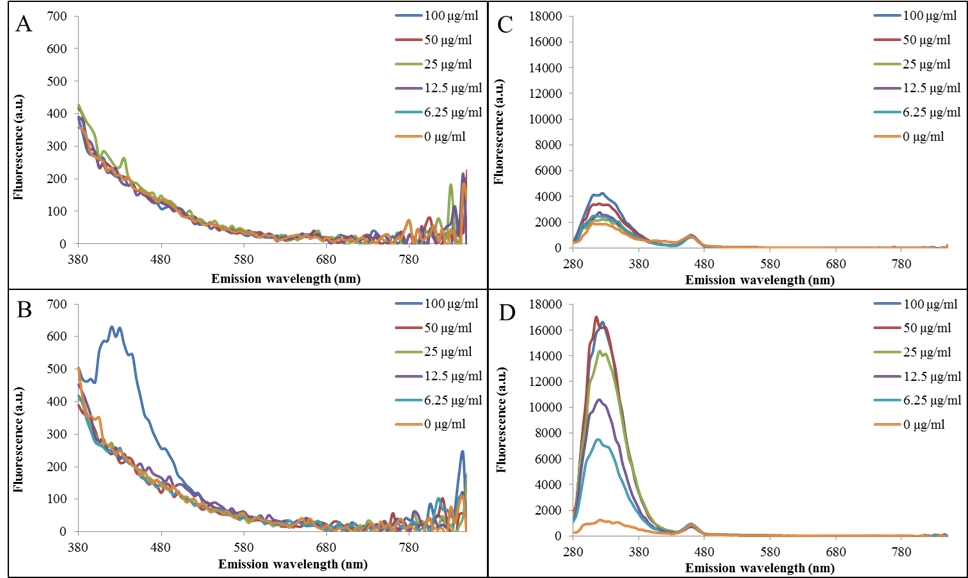
Figure 3: Modification of anti-DNA antibodies with MWCNT-CQDs determined by ELISA-like method with fluorescent detection. (A) Fluorescence spectra (excitation wavelength 330 nm) of microtiter plate wells coated with DNA fragment after incubation with anti-ssDNA antibodies. (B) Fluorescence spectra (excitation wavelength 330 nm) of microtiter plate wells coated with DNA fragment after incubation with anti-ssDNA antibodies modified with MWCNT-CQDs. (C) Fluorescence spectra (excitation wavelength 230 nm) of microtiter plate wells coated with DNA fragment after incubation with anti-ssDNA antibodies. (D) Fluorescence spectra (excitation wavelength 230 nm) of microtiter plate wells coated with DNA fragment after incubation with anti-ssDNA antibodies modified with MWCNT-CQDs.
1. Durandy, A.; Kracker, S. Immunoglobulin class-switch recombination deficiencies. Arthritis Res. Ther. 2012, 14.
2. Zhao, Y.; Rabbani, H.; Shimizu, A.; Hammarstrom, L. Mapping of the chicken immunoglobulin heavy-chain constant region gene locus reveals an inverted alpha gene upstream of a condensed upsilon gene. Immunology 2000, 101, 348-353.
3. Janu, L.; Stanisavljevic, M.; Krizkova, S.; Sobrova, P.; Vaculovicova, M.; Kizek, R.; Adam, V. Electrophoretic study of peptide-mediated quantum dot-human immunoglobulin bioconjugation. Electrophoresis 2013, 34, 2725-2732.
4. Lin, J.; Wang, R.; Jiao, P.; Li, Y.; Li, Y.; Liao, M.; Yu, Y.; Wang, M. An impedance immunosensor based on low-cost microelectrodes and specific monoclonal antibodies for rapid detection of avian influenza virus h5n1 in chicken swabs. Biosensors & bioelectronics 2015, 67, 546-552.
5. Li, W.; Jiang, X.; Xue, J.; Zhou, Z.; Zhou, J. Antibody modified gold nano-mushroom arrays for rapid detection of alpha-fetoprotein. Biosensors & bioelectronics 2015, 68, 468-474.
6. Weng, C.-I.; Chang, H.-T.; Lin, C.-H.; Shen, Y.-W.; Unnikrishnan, B.; Li, Y.-J.; Huang, C.-C. One-step synthesis of biofunctional carbon quantum dots for bacterial labeling. Biosensors & bioelectronics 2015, 68, 1-6.
7. Krizkova, S.; Nguyen, H.V.; Stanisavljevic, M.; Kopel, P.; Vaculovicova, M.; Adam, V.; Kizek, R. Microchip capillary electrophoresis: Quantum dots and paramagnetic particles for bacteria immunoseparation : Rapid superparamagnetic-beads-based automated immunoseparation of zn-proteins from staphylococcus aureus with nanogram yield. Methods in molecular biology (Clifton, N.J.) 2015, 1274, 67-79.
8. Zitka, O.; Krizkova, S.; Skalickova, S.; Dospivova, D.; Adam, V.; Kizek, R. Microfluidic tool coupled with electrochemical assay for detection of lactoferrin isolated by antibody-modified paramagnetic beads. Electrophoresis 2013, 34, 2120-2128.
9. Krizkova, S.; Ryvolova, M.; Hynek, D.; Eckschlager, T.; Hodek, P.; Masarik, M.; Adam, V.; Kizek, R. Immunoextraction of zinc proteins from human plasma using chicken yolk antibodies immobilized onto paramagnetic particles and their electrophoretic analysis. Electrophoresis 2012, 33, 1824-1832.
10. Ceppellini, R.; Polli, E.; Celada, F. A DNA-reacting factor in serum of s patient with lupus erythematosus diffusus. Proc. Soc. Exp. Biol. Med. 1957, 96, 572-574.
11. Schur, P.H.; Sandson, J. Immunologic factors and clinical activity in systemic lupus erythematosus N. Engl. J. Med. 1968, 278, 533-&.
12. Tojo, T.; Friou, G.J. Lupus nephritis - varying complement-fixing properties od immunoglobulin g antibodies to antigens of cell nuclei Science 1968, 161, 904-&.
13. Stearns, N.A.; Lee, J.; Leong, K.W.; Sullenger, B.A.; Pisetsky, D.S. The inhibition of anti-DNA binding to DNA by nucleic acid binding polymers. PLoS One 2012, 7.
14. Rothfiel.Nf; Stollar, B.D. Relation of immunoglobulin class pattern of antinuclear antibody and complement-fixing antibodies to DNA in sera from patients with systemic lupus erythematosus J. Clin. Invest. 1967, 46, 1785-&.
15. Oka, Y.; Hirabayashi, Y.; Takahashi, R.; Ishii, T.; Sasaki, T. Can viral infection be a trigger of anti-DNA antibody production through endoplasmic reticulum stress? Arthritis Rheum. 2006, 54, S298-S298.
16. Kalsi, J.K.; Martin, A.C.R.; Hirabayashi, Y.; Ehrenstein, M.; Longhurst, C.M.; Ravirajan, C.; Zvelebil, M.; Stollar, B.D.; Thornton, J.M.; Isenberg, D.A. Functional and modelling studies of the binding of human monoclonal anti-DNA antibodies to DNA. Mol. Immunol. 1996, 33, 471-+.
17. Hirose, J.; Inoue, K.; Morimoto, E.; Iwamoto, H.; Yamaguti, Y.; Kitase, M.; Inagaki, K.; Hiromi, K. Characterization of monoclonal antibodies against (1r,2r)-cyclohexanediamine platinum(ii)-DNA adduct. Biol. Pharm. Bull. 1996, 19, 1220-1222.
18. Xia, Y.M.; Janda, A.; Eryilmaz, E.; Casadevall, A.; Putterman, C. The constant region affects antigen binding of antibodies to DNA by altering secondary structure. Mol. Immunol. 2013, 56, 28-37.
19. Stanisavljevic, M.; Chomoucka, J.; Dostalova, S.; Krizkova, S.; Vaculovicova, M.; Adam, V.; Kizek, R. Interactions between cdte quantum dots and DNA revealed by capillary electrophoresis with laser-induced fluorescence detection. Electrophoresis 2014, 35, 2587-2592.
20. Xiao, J.; Liu, P.; Li, L.H.; Yang, G.W. Fluorescence origin of nanodiamonds. J. Phys. Chem. C 2015, 119, 2239-2248.
21. Du, F.Y.; Li, J.A.; Hua, Y.; Zhang, M.M.; Zhou, Z.; Yuan, J.; Wang, J.; Peng, W.X.; Zhang, L.; Xia, S., et al. Multicolor nitrogen-doped carbon dots for live cell imaging. J. Biomed. Nanotechnol. 2015, 11, 780-788.
22. Pandey, N.; Srivastava, R.K.; Singh, M.K.; Singh, J. Optical properties of carbon nanodots synthesized by laser induced fragmentation of graphite powder suspended in water. Mater. Sci. Semicond. Process 2014, 27, 150-153.
23. Soldano, C. Hybrid metal-based carbon nanotubes: Novel platform for multifunctional applications. Prog. Mater. Sci. 2015, 69, 183-212.
24. Hou, J.; Dong, J.; Zhu, H.; Teng, X.; Ai, S.; Mang, M. A simple and sensitive fluorescent sensor for methyl parathion based on l-tyrosine methyl ester functionalized carbon dots. Biosensors & bioelectronics 2015, 68, 20-26.
25. Chen, X.F.; Zhang, W.X.; Wang, Q.J.; Fan, J.Y. C-8-structured carbon quantum dots: Synthesis, blue and green double luminescence, and origins of surface defects. Carbon 2014, 79, 165-173.
26. Milosavljevic, V.; Nguyen, H.V.; Michalek, P.; Moulick, A.; Kopel, P.; Kizek, R.; Adam, V. Synthesis of carbon quantum dots for DNA labeling and its electrochemical, fluorescent and electrophoretic characterization. Chem. Pap. 2015, 69, 192-201.
27. Krizkova, S.; Adam, V.; Eckschlager, T.; Kizek, R. Using of chicken antibodies for metallothionein detection in human blood serum and cadmium-treated tumour cell lines after dot- and electroblotting. Electrophoresis 2009, 30, 3726-3735.
J.Met.Nano:
volume-2, issue-3
- Laboratory of Metallomics and Nanotechnologies – an initiator of the Metallomics Scientific Network formation
- Capillary electrophoresis of metallothionein
- Synthetic birnessites and buserites as heavy metal cation traps and environmental remedies
- Immunohistochemical detection of metallothionein
- MALDI-TOF MSI and electrochemical detection of metallothionein in chicken liver after cadmium exposure
- The use of MALDI MSI for the study of different tissues
- Utilization of graphene oxide electrophoretic deposition for construction of electrochemical sensors and biosensors
- Influence of Different Inducers on Ligninolytic Enzyme Activities
- Interaction of nanocarrier apoferritin with cytotoxic drug molecules
- Study of cell penetrating peptide and Europium(III) and Terbium(III) Schiff base complexes interaction
- HPV Detection in Leukocyte Samples of Spinocellular Carcinomas Using PCR
- Characterization of carbon quantum dots by capillary electrophoresis with laser-induced fluorescence detections
- Modification of anti-DNA antibodies with carbon quantum dots
- Fluorescence detection of carbon quantum dots assessed by stratospheric platform
 PDF
PDF