
Interaction of nanocarrier apoferritin with cytotoxic drug molecules
Simona Dostalova, Monica Vazzana, Marketa Vaculovicova, Vojtech Adam and Rene Kizek
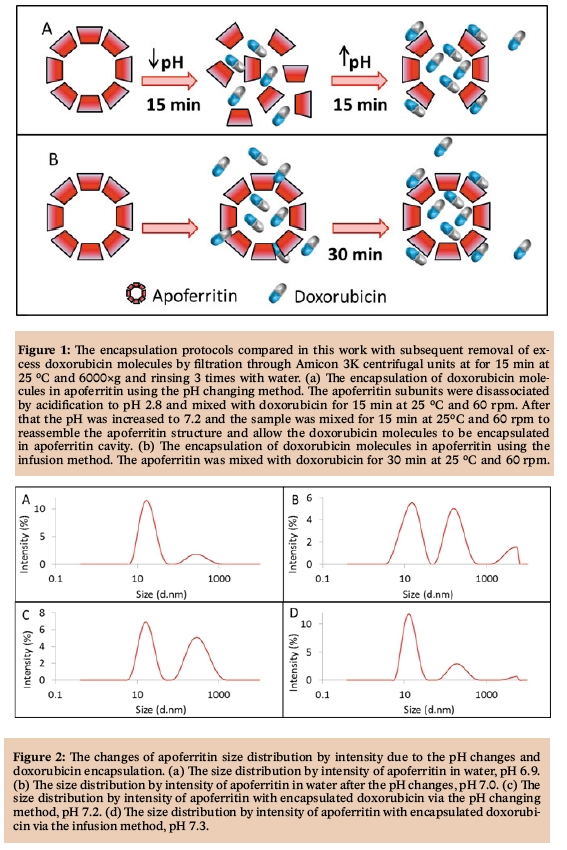
Naturally occurring or artificially prepared proteins can be employed in various fields including basic research [1-4], industry [5-8], immuno-based assays [9-12], detection of many different analytes [13-16], conventional medicine [17-20] or nanomedicine [21-24]. The first nanomedicine formulation ever approved by the FDA was a PEGylated bovine serum albumin in 1990, named Adagen® [25]. Out of the 44 FDA-approved nanomedicine formulations, 10 are polymer-protein conjugates or albumin nanoparticles [26-28] and novel protein-based nanocarriers are still being developed [29-32]. The use of proteins as nanocarriers has many advantages in comparison with artificial nanocarriers [33]. The immune response of patient’s body to protein nanocarriers is much lower, especially for the proteins naturally occurring in humans or after surface modification with polyethyleneglycol [34]. Moreover, they can usually self-assemble to form uniform cages with an extraordinary binding capacity for various drugs. They are also biodegradable, and have abundant renewable sources [35]. The high binding capacity of protein nanocarriers owns to multiple functional groups in their primary sequence allowing for different interactions with therapeutic molecules [36]. Drug molecules can also be reversibly encapsulated in some proteins’ three-dimensional hollow cage [37]. The most frequently studied protein as a nanocarrier is a human serum albumin, the most abundant protein in blood plasma (about 60% of the total plasma protein count) [38]. Albumin is a globular protein containing 585 amino acids (with one tryptophan residue) and three homologous domains stabilized by disulfide bridges [39]. The ligands, either organic or inorganic, usually bind to the many hydrophobic cavities in albumin subdomains, making this protein an important regulator of intercellular fluxes and, moreover, drug behavior in vivo [40]. Ligands can interact with both C=O and C-N albumin groups, resulting in changes in its secondary structure [41]. Albumin nanoparticles were used for delivery of cytostatic drugs, siRNAs, vaccines or radiodiagnostics [42-45]. Protein apoferritin is also studied as a possible nanocarrier [29,46-48]. Apoferritin is also naturally found in human body as a major iron-binding protein. Apoferritin forms a hollow rhombic dodecahedral shell with outer diameter of 12-13 nm and an inner diameter of 7-8 nm [49]. The ligands can thus not only interact with amino acid residues of apoferritin but also be encapsulated in its hollow cavity. Many amino acid side chains of apoferritin 24 subunits interact to form hydrophobic cores, but there is also a large number of polar and hydrophilic residues, allowing for interaction with various molecules [50]. Some substances can bind to the apoferritin surface through hydrogen bonding (non-ionic molecules) or electrostatic interactions (ionic molecules) [51]. The apoferritin shell contains both hydrophobic and hydrophilic channels, allowing small molecules to enter the cavity via diffusion [52]. Its subunits can also be reversibly disassociated via the change of surrounding environment to encapsulate bigger molecules after reassembling [53]. In this work, we studied the interactions of cytotoxic drug doxorubicin with the apoferritin protein. Two encapsulation methods were compared, the pH changing and infusion method.
![Figure 1: Lecture by Associate Professor Vojtech
Adam of Mendel University in Brno on the role of
metallothionein in cancer [1]. houbova](pics/d3.jpg)
![Figure 1: Lecture by Associate Professor Vojtech
Adam of Mendel University in Brno on the role of
metallothionein in cancer [1]. houbova](pics/d5.jpg)
1. Chayaratanasin, P.; Barbieri, M.A.; Suanpairintr, N.; Adisakwattana, S. Inhibitory effect of clitoria ternatea flower petal extract on fructose-induced protein glycation and oxidation-dependent damages to albumin in vitro. BMC complementary and alternative medicine 2015, 15, 546.
2. Kim, K.-W.; Kim, B.-M.; Moon, H.-W.; Lee, S.-H.; Kim, H.-R. Role of c-reactive protein in osteoclastogenesis in rheumatoid arthritis. Arthritis research & therapy 2015, 17, 563.
3. Moran, G.; Carcamo, C.; Concha, M.; Folch, H. Expression of the protein serum amyloid a in response to aspergillus fumigatus in murine models of allergic airway inflammation. Rev. Iberoam. Micol. 2015, 32, 25-29.
4. Tsushima, H.; Okazaki, K.; Ishihara, K.; Ushijima, T.; Iwamoto, Y. Ccaat/enhancer-binding protein beta promotes receptor activator of nuclear factor-kappa-b ligand (rankl) expression and osteoclast formation in the synovium in rheumatoid arthritis. Arthritis research & therapy 2015, 17, 532.
5. Fujiwara, M.; Imura, T. Transparent silica thin films prepared from sodium silicate and bovine serum albumin with petal effect. Ceram. Int. 2015, 41, 7565-7572.
6. Rasala, B.A.; Mayfield, S.P. Photosynthetic biomanufacturing in green algae; production of recombinant proteins for industrial, nutritional, and medical uses. Photosynth. Res. 2015, 123, 227-239.
7. Zhao, Y.; Zhao, Y.Z.; Xu, H.L.; Yang, Y.Q. A sustainable slashing industry using biodegradable sizes from modified soy protein to replace petro-based poly(vinyl alcohol). Environ. Sci. Technol. 2015, 49, 2391-2397.
8. Zhao, Y.Z.; Zhao, Y.; Yang, Y.Q. Modified soy protein to substitute non-degradable petrochemicals for slashing industry. Ind. Crop. Prod. 2015, 67, 466-474.
9. Heger, Z.; Cernei, N.; Krizkova, S.; Masarik, M.; Kopel, P.; Hodek, P.; Zitka, O.; Adam, V.; Kizek, R. Paramagnetic nanoparticles as a platform for fret-based sarcosine picomolar detection. Sci Rep 2015, 5.
10. Krizkova, S.; Ryvolova, M.; Hynek, D.; Eckschlager, T.; Hodek, P.; Masarik, M.; Adam, V.; Kizek, R. Immunoextraction of zinc proteins from human plasma using chicken yolk antibodies immobilized onto paramagnetic particles and their electrophoretic analysis. Electrophoresis 2012, 33, 1824-1832.
11. Van Zoelen, S.A.; Ozkan, O.; Inceoglu, B. Antigenic cross-reactivity anti-birtoxin antibody against androctonus crassicauda venom. J. Arthropod.-Borne Dis. 2015, 9, 176-183.
12. Zitka, O.; Krizkova, S.; Skalickova, S.; Dospivova, D.; Adam, V.; Kizek, R. Microfluidic tool coupled with electrochemical assay for detection of lactoferrin isolated by antibody-modified paramagnetic beads. Electrophoresis 2013, 34, 2120-2128.
13. Abdelwahab, M.; Loa, C.C.; Wu, C.C.; Lin, T.L. Recombinant nucleocapsid protein-based enzyme-linked immunosorbent assay for detection of antibody to turkey coronavirus. J. Virol. Methods 2015, 217, 36-41.
14. Heger, Z.; Zitka, O.; Fohlerova, Z.; Rodrigo, M.A.M.; Hubalek, J.; Kizek, R.; Adam, V. Use of green fluorescent proteins for in vitro biosensing. Chem. Pap. 2015, 69, 54-61.
15. Minnaard, M.C.; Van De Pol, A.C.; De Groot, J.A.H.; De Wit, N.J.; Hopstaken, R.M.; Van Delft, S.; Goossens, H.; Ieven, M.; Lammens, C.; Little, P., et al. The added diagnostic value of five different c-reactive protein point-of-care test devices in detecting pneumonia in primary care: A nested case-control study. Scand. J. Clin. Lab. Invest. 2015, 75, 291-295.
16. Sonaimuthu, P.; Cheong, F.W.; Chin, L.C.; Mahmud, R.; Fong, M.Y.; Lau, Y.L. Detection of human malaria using recombinant plasmodium knowlesi merozoire surface protein-1 (msp-119) expressed in escherichia coli. Experimental parasitology 2015, 153, 118-122.
17. Kassir, R.; Blanc, P.; Tibalbo, L.M.B.; Breton, C.; Lointier, P. C-reactive protein and procalcitonin for the early detection of postoperative complications after sleeve gastrectomy: Preliminary study in 97 patients. Surg. Endosc. 2015, 29, 1439-1444.
18. Liyasova, M.S.; Ma, K.; Lipkowitz, S. Molecular pathways: Cbl proteins in tumorigenesis and antitumor immunity-opportunities for cancer treatment. Clin. Cancer Res. 2015, 21, 1789-1794.
19. Numbenjapon, N.; Chamnanwanakij, S.; Sangaroon, P.; Simasathien, S.; Watanaveeradej, V. C-reactive protein as a single useful parameter for discontinuation of antibiotic treatment in thai neonates with clinical sepsis. Journal of the Medical Association of Thailand = Chotmaihet thangphaet 2015, 98, 352-357.
20. Zali, H.; Zamanian-Azodi, M.; Tavirani, M.R.; Baghban, A.A.Z. Protein drug targets of lavandula angustifolia on treatment of rat alzheimer‘s disease. Iran. J. Pharm. Res. 2015, 14, 291-302.
21. Heger, Z.; Cernei, N.; Blazkova, I.; Kopel, P.; Masarik, M.; Zitka, O.; Adam, V.; Kizek, R. Gamma-fe2o3 nanoparticles covered with glutathione-modified quantum dots as a fluorescent nanotransporter. Chromatographia 2014, 77, 1415-1423.
22. Janu, L.; Stanisavljevic, M.; Krizkova, S.; Sobrova, P.; Vaculovicova, M.; Kizek, R.; Adam, V. Electrophoretic study of peptide-mediated quantum dot-human immunoglobulin bioconjugation. Electrophoresis 2013, 34, 2725-2732.
23. Rodrigues, S.; Cordeiro, C.; Seijo, B.; Remunan-Lopez, C.; Grenha, A. Hybrid nanosystems based on natural polymers as protein carriers for respiratory delivery: Stability and toxicological evaluation. Carbohydr. Polym. 2015, 123, 369-380.
24. Sahebalzamani, M.; Biazar, E.; Shahrezaei, M.; Hosseinkazemi, H.; Rahiminavaie, H. Surface modification of phbv nanofibrous mat by laminin protein and its cellular study. Int. J. Polym. Mater. Polym. Biomat. 2015, 64, 149-154.
25. Syiam, M.M.; El-Aziem, M.A.; Soliman, M.E.M. Adagen: Adaptive interface agent for x-ray fracture detection. Ieee: New York, 2004; p 354-357.
26. Alconcel, S.N.S.; Baas, A.S.; Maynard, H.D. Fda-approved poly(ethylene glycol)-protein conjugate drugs. Polym. Chem. 2011, 2, 1442-1448.
27. Gommans, G.M.M.; Boer, R.O.; van Dongen, B.A.; van der Schors, T.G.; de Waard, J.W.D. Optimisation of 99mtc-nanocoll sentinel node localisation in carcinoma of the breast. Eur. J. Nucl. Med. 2000, 27, 744-744.
28. O‘Shaughnessy, J.A.; Blum, J.L.; Sandbach, J.F.; Savin, M.; Fenske, E.; Hawkins, M.J.; Baylor-Charles, A. Weekly nanoparticle albumin paclitaxel (abraxane) results in long-term disease control in patients with taxane-refractory metastatic breast cancer. Breast Cancer Res. Treat. 2004, 88, S65-S65.
29. Blazkova, I.; Nguyen, V.H.; Dostalova, S.; Kopel, P.; Stanisavljevic, M.; Vaculovicova, M.; Stiborova, M.; Eckschlager, T.; Kizek, R.; Adam, V. Apoferritin modified magnetic particles as doxorubicin carriers for anticancer drug delivery. Int. J. Mol. Sci. 2013, 14, 13391-13402.
30. Chen, C.H.; Hu, H.Y.; Qiao, M.X.; Zhao, X.L.; Wang, Y.J.; Chen, K.; Chen, D.W. Anti-tumor activity of paclitaxel through dual-targeting lipoprotein-mimicking nanocarrier. J. Drug Target. 2015, 23, 311-322.
31. Mottaghitalab, F.; Farokhi, M.; Shokrgozar, M.A.; Atyabi, F.; Hosseinkhani, H. Silk fibroin nanoparticle as a novel drug delivery system. J. Control. Release 2015, 206, 161-176.
32. Zhou, Z.L.; Badkas, A.; Stevenson, M.; Lee, J.Y.; Leung, Y.K. Herceptin conjugated plga-phis-peg ph sensitive nanoparticles for targeted and controlled drug delivery. Int. J. Pharm. 2015, 487, 81-90.
33. Dostalova, S.; Munzova, D.; Vaculovicova, M.; Kizek, R. Delivery of doxorubicin using protein nanocarriers. J. Metallomics Nanotech. 2014, 1, 34-38.
34. Ma, Y.J.; Nolte, R.J.M.; Cornelissen, J. Virus-based nanocarriers for drug delivery. Adv. Drug Deliv. Rev. 2012, 64, 811-825.
35. Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Protein-based nanocarriers as promising drug and gene delivery systems. J. Control. Release 2012, 161, 38-49.
36. Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release 2012, 157, 168-182.
37. Elzoghby, A.O.; El-Fotoh, W.S.A.; Elgindy, N.A. Casein-based formulations as promising controlled release drug delivery systems. J. Control. Release 2011, 153, 206-216.
38. Chen, T.; Zhu, X.; Chen, Q.; Ge, M.; Jia, X.; Wang, X.; Ge, C. Interaction between z-ligustilide from radix angelica sinensis and human serum albumin. Food Chem. 2015, 186, 292-297.
39. Sugio, S.; Kashima, A.; Mochizuki, S.; Noda, M.; Kobayashi, K. Crystal structure of human serum albumin at 2.5 angstrom resolution. Protein Eng. 1999, 12, 439-446.
40. Varshney, A.; Rehan, M.; Subbarao, N.; Rabbani, G.; Khan, R.H. Elimination of endogenous toxin, creatinine from blood plasma depends on albumin conformation: Site specific uremic toxicity & impaired drug binding. PLoS One 2011, 6.
41. Liu, Y.; Chen, M.M.; Jiang, L.G.; Song, L. Stereoselective interaction of cinchona alkaloid isomers with bovine serum albumin. Food Chem. 2015, 181, 170-178.
42. Kunda, N.K.; Alfagih, I.M.; Dennison, S.R.; Tawfeek, H.M.; Somavarapu, S.; Hutcheon, G.A.; Saleem, I.Y. Bovine serum albumin adsorbed pga-co-pdl nanocarriers for vaccine delivery via dry powder inhalation. Pharm. Res. 2015, 32, 1341-1353.
43. Sharma, R.I.; Pereira, M.; Schwarzbauer, J.E.; Moghe, P.V. Albumin-derived nanocarriers: Substrates for enhanced cell adhesive ligand display and cell motility. Biomaterials 2006, 27, 3589-3598.
44. Son, S.; Song, S.; Lee, S.J.; Min, S.; Kim, S.A.; Yhee, J.Y.; Huh, M.S.; Kwon, I.C.; Jeong, S.Y.; Byun, Y., et al. Self-crosslinked human serum albumin nanocarriers for systemic delivery of polymerized sirna to tumors. Biomaterials 2013, 34, 9475-9485.
45. Yuan, A.; Wu, J.H.; Song, C.C.; Tang, X.L.; Qiao, Q.; Zhao, L.L.; Gong, G.M.; Hu, Y.Q. A novel self-assembly albumin nanocarrier for reducing doxorubicin-mediated cardiotoxicity. J. Pharm. Sci. 2013, 102, 1626-1635.
46. Heger, Z.; Skalickova, S.; Zitka, O.; Adam, V.; Kizek, R. Apoferritin applications in nanomedicine. Nanomedicine 2014, 9, 2233-2245.
47. Munzova, D.; Dostalova, S.; Vaculovicova, M.; Kizek, R. Apoferritin: Nanocarrier for targeted drug delivery. J. Metallomics Nanotech. 2014, 1, 50-54.
48. Tmejova, K.; Hynek, D.; Kopel, P.; Dostalova, S.; Smerkova, K.; Stanisavljevic, M.; Nguyen, V.H.; Nejdl, L.; Vaculovicova, M.; Krizkova, S., et al. Electrochemical behaviour of doxorubicin encapsulated in apoferritin. Int. J. Electrochem. Sci. 2013, 8, 12658-12671.
49. Haussler, W. Structure and dynamics in apoferritin solutions with paracrystalline order. Chem. Phys. 2003, 292, 425-434.
50. Crichton, R.R.; Declercq, J.P. X-ray structures of ferritins and related proteins. Biochim. Biophys. Acta-Gen. Subj. 2010, 1800, 706-718.
51. Liu, F.; Du, B.J.; Chai, Z.; Zhao, G.H.; Ren, F.Z.; Leng, X.J. Binding properties of apoferritin to nicotinamide and calcium. Eur. Food Res. Technol. 2012, 235, 893-899.
52. Granier, T.; Gallois, B.; Dautant, A.; Destaintot, B.L.; Precigoux, G. Comparison of the structures of the cubic and tetragonal forms of horse-spleen apoferritin. Acta Crystallogr. Sect. D-Biol. Crystallogr. 1997, 53, 580-587.
53. Kim, M.; Rho, Y.; Jin, K.S.; Ahn, B.; Jung, S.; Kim, H.; Ree, M. Ph-dependent structures of ferritin and apoferritin in solution: Disassembly and reassembly. Biomacromolecules 2011, 12, 1629-1640.
54. Konecna, R.; Nguyen, H.V.; Stanisavljevic, M.; Blazkova, I.; Krizkova, S.; Vaculovicova, M.; Stiborova, M.; Eckschlager, T.; Zitka, O.; Adam, V., et al. Doxorubicin encapsulation investigated by capillary electrophoresis with laser-induced fluorescence detection. Chromatographia 2014, 77, 1469-1476.
J.Met.Nano:
volume-2, issue-3
- Laboratory of Metallomics and Nanotechnologies – an initiator of the Metallomics Scientific Network formation
- Capillary electrophoresis of metallothionein
- Synthetic birnessites and buserites as heavy metal cation traps and environmental remedies
- Immunohistochemical detection of metallothionein
- MALDI-TOF MSI and electrochemical detection of metallothionein in chicken liver after cadmium exposure
- The use of MALDI MSI for the study of different tissues
- Utilization of graphene oxide electrophoretic deposition for construction of electrochemical sensors and biosensors
- Influence of Different Inducers on Ligninolytic Enzyme Activities
- Interaction of nanocarrier apoferritin with cytotoxic drug molecules
- Study of cell penetrating peptide and Europium(III) and Terbium(III) Schiff base complexes interaction
- HPV Detection in Leukocyte Samples of Spinocellular Carcinomas Using PCR
- Characterization of carbon quantum dots by capillary electrophoresis with laser-induced fluorescence detections
- Modification of anti-DNA antibodies with carbon quantum dots
- Fluorescence detection of carbon quantum dots assessed by stratospheric platform
 PDF
PDF