
Capillary electrophoresis of metallothionein
Marta Kepinska* and Halina Milnerowicz
Metallothionein (MT) was first isolated in 1957 from the horse kidney by Margoshes and Vallee [1], subsequently MT presence was demonstrated in other animals, in higher plants, eukaryotic microorganisms, and in some prokaryotes [2]. Mammalian MT belongs to a group of low molecular weight proteins (6000-7000 Da) with 30% content of cysteine. A characteristic feature of all MTs is a tripeptide sequence Cys-Xaa-Cys, where Xaa is an aminoacid other than cysteine [3]. The sulfhydryl groups of cysteine form with metals closely packed spatial structure in which metals are within the molecule [2,4]. It serves as the reservoir of metals for the body (mainly Zn and Cu) which are the part of many enzymes and proteins involved in the removal of DNA damage, replication or transcription. MT protects the cells against the toxicity of heavy metals (Cd, Pb, Hg) by binding them. MT is composed of two domains: α and β linked by lysine dimer. Domain α (C-terminal) is more stable and is able to bind four atoms of Zn or Cd, or five or six atoms of Cu. Beta domain (N terminal) is more reactive, it can bind tri- Zn or Cd, or six atoms of Cu [4]. Slight differences in amino acid composition, hydrophobicity and isoelectric point allowed to separate the four major isoforms: MT-1, MT-2, MT-3 (also known as, growth inhibitory factor GIF), and MT-4. Despite the mutual similarity of MT-1 and MT-2, their roles and presence in tissues differ significantly [5]. While MT-2 isoform in humans is encoded by a single gene MT-2A, MT-1 consists of multiple subtypes encoded by a gene set of MT-1 (MT-1A, 1B-MT, MT-1E, MT-1F, MT-1G, 1H and MT-MT-1X), which determine the micro-heterogeneity of the protein (Figure 1).
< p class="juxe"> MT-1 and MT-2 genes expression have been demonstrated in many tissues: in the parenchymal cells of the kidney, liver, lung, intestines, pancreas [7], in cells of the sweat glands of the skin, in germ cells, and in epithelial cells of the thymus [8]. Izofom MT-3 is a protein mainly present in brain tissue [9]. The expression of MT-4 is limited to squamous skin and upper gastrointestinal [10]. Sites of presence of particular isoforms of human MT are listed in Table 1 [11]. MT cysteine residues also play a critical role in the removal of free radicals. MT has antitumor activity as it neutralizes toxic electrophiles, reactive oxygen and nitrogen species. MT is also capable of inhibiting apoptosis and developing resistance to radiotherapy and chemotherapy. Higher expression of this protein has been observed in many tumor tissues: lung, kidney, pancreas, bladder, urinary tract. Lower concentration levels have been observed in gastrointestinal tumors tract, including liver cancer or colon cancer. < p class="juxe"> To identify MT, sensitive and selective analysis techniques are applied. Low molecular weight of protein, and the heterogeneity of the biological material to be analyzed (serum, erythrocyte lysate, urine, different tissues), also causes a variety of methods used for its quantitative determination. < p class="juxe"> Electrochemical methods such as differential pulse and cathodic stripping voltammetry, saturation analysis methods based on Cd, Ag and Hg, spectrophotometric methods as well as chromatographic and electrophoretic techniques are used [12]. Among immunological techniques used in MT analyses are: enzyme-linked immunosorbent assay, immunofluorescence assay and radioimmunoassay. These methods are highly sensitive and can detect even small amounts of MT in tested material [13,14]. One of the most widely used techniques in the analysis of proteins including MT is two-dimensional polyacrylamide gel electrophoresis or capillary electrophoresis (CE) often coupled with mass spectrometry (MS). CE allows analysis of both MT as MT in complexes with metals. As the determination of various isoforms is concerned, there must be very sophisticated detection system connected with separation one. Today, medicine has a lot of interest in research on this protein, and thanks to modern analytical methods with high sensitivity and specificity, the determination of MT concentrations in the biological material is possible, wherein its amount in relation to other proteins is small.![Figure 1: Lecture by Associate Professor Vojtech
Adam of Mendel University in Brno on the role of
metallothionein in cancer [1]. houbova](pics/kepinska-1.jpg)
Figure 1: Comparison of the amino acid sequences of MT 1/2 human isoforms using the ClustalX program 2.0.11 [6].
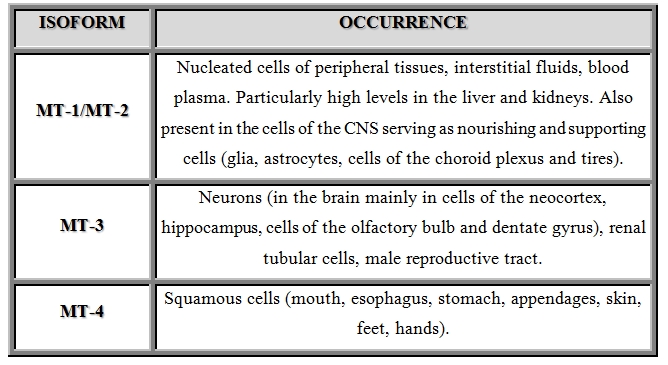
Table 1: Places of expression of particular isoforms of human MT [Based on 11].
1.Margoshes, M.; Vallee, B.L. A cadmium protein from equine kidney cortex. J. Am. Chem. Soc. 1957,79, 4813-4814.
2. Klaassen, C.D.; Liu, J.; Chaudhuri, S. Metallothionein: an intracellular protein to protect against cadmium toxicity. Ann. Rev. Pharmacol. Toxicol. 1999, 39, 267-94.
3. Dabrio, M.; Rodriquez, A.R.; Bordin, G.; Bebianno, M.J.; Ley M.D.; Sestakova, I.; Vasak, M.; Nordberg, M. Recent developments in quantification methods for metallothionein. J. Inorg. Biochem. 2002, 88, 123-134.
4. Klaassen, C.D.; Liu, J. Role of metallothionein in cadmium-induced hepatotoxicity and nephrotoxicity. Drug Metab. Rev. 1997, 29, 79-102.
5. Zalewska, M.; Trefon, J.; Milnerowicz, H. The role of metallothionein interactions with other proteins. Proteomics. 2014, 14, 1343-56. Review.
6. Thompson, J.; Higgins, D.; Gibson, T. CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nuc. Acids Res. 1994, 22, 4673-4680.
7. Lynes, M.A.; Borghesi, L.A.; Youn, J.; Olson, E.A. Immunomodulatory activities of extracellular metallothionein. I. Metallothionein effects on antibody production. Toxicology 1993, 85,161-177.
8. Vandenoord, J.J.; Deley, M. Distribution of metallothionein in normal and pathological human skin. Arch. Dermatol. Res. 1994, 286, 62-68.
9. Uchida, Y.; Takio, K.; Titani, K.; Ihara, Y.; Tomonaga, M. The growth inhibitory factor that is deficient in the Alzheimer‘s disease brain is a 68 amino acid metallothionein-like protein. Neuron 1991, 7, 337-47.
10. Quaife, C.J.; Findley, S.D.; Erickson, J.C. Induction of a new metallothionein isoforms (MT-IV) occurs during differentiation of stratified epithelia. Biochemistry 1994 ,33, 7250-7259.
11. Ryvolova, M.; Krizkowa, S.; Adam, V.; Becklova, M.; Trnkova, L.; Hubalek, J.; Kizek, R. Analytical methods for metallothionein detection. Curr. Anal. Chem. 2011, 7, 243-261.
12. Ryvolova, M.; Adam, V.; Kizek, R. Analysis of metallothionein by capillary electrophoresis. J Chromatogr A 2012, 1226, 31-42. Review.
13. Milnerowicz, H.; Bizoń, A. Determination of metallothionein in biological fluids using enzyme-linked immunoassay with commercial antibody. Acta Biochim Pol. 2010, 57, 99-104.
14. Krizkova, S.; Ryvolova, M.; Masarik, M.; Zitka, O.; Adam, V.; Hubalek, J.; Eckschlager, T.; Kizek, R. Modern bioanalysis of proteins by electrophoretic techniques. Methods Mol Biol. 2014, 1129, 381-396.
15. Zalewska, M.; Bizoń, A.; Milnerowicz, H. Comparison of capillary electrophoretic techniques for analysis and characterization of metallothioneins. J Sep Sci. 2011, 34, 3061-3069.
16. Sieradzka, E.; Witt, K.; Milnerowicz, H. The application of capillary electrophoresis techniques in toxicological analysis. Biomed Chromatogr. 2014, 28, 1507-1513. Review.
17. Beattie, J.H.; Richards, M.P. Analysis of metallothionein isoforms by capillary electrophoresis: optimization of protein separation conditions using micellar electrokinetic capillary chromatography. J Chromatogr A. 1995, 700, 95-103
18. Bo, T.; Pawliszyn, J. Characterization of phospholipid-protein interactions by capillary isoelectric focusing with whole-column imaging detection. Anal Biochem. 2006, 350, 91-98.
19. Riekkola, M.L.; Jonsson, J.A.; Smith, R.M. Terminology for analytical capillary electromigration. Pure Appl. Chem. 2004, 76, 443-451
20. Ding S.; Qi. L.; Tian K. Novel and simple nonaqueous capillary electrophoresis separation and determination bioactive triterpens in Chinese herbs. J. Pharm. Biomed. Anal. 2006, 40, 35-41.
21. Richards, M.P.; Beattie, J.H. Comparison of different techniques for the analysis of metallothionein isoforms by capillary electrophoresis. J. Chromatogr. B. 1995, 669, 27-37.
22. Beattie, J.H. Strategies for the qualitative and quantitative analysis of metallothionein isoforms by capillary electrophoresis. Talanta 1998, 46, 255-270.
23. Li, Y.; Yang, H.; Liu, N.; Luo, J.; Wang, Q.; Wang, L. Cadmium accumulation and metallothionein biosynthesis in cadmium-treated freshwater mussel Anodonta woodiana. PLoS One 2015, 10, e0117037.
24. Kubo, K.; Sakita, Y.; Minami, T. Effect of heat treatment on metallothionein isoforms using capillary zone electrophoresis. Analusis 2000, 28, 366-369.
25. Virtanen, V.; Bordin, G.; Rodriguez, A.R. Separation of metallothionein isoforms with capillary zone electrophoresis using an uncoated capillary column—effects of pH, temperature, voltage, buffer concentration and buffer composition. J Chromatogr A 1996, 734, 391-400.
26. Virtanen, V.; Bordin, G. Tricine buffer for metallothionein isoform separation by capillary zone electrophoresis. Anal Chim Acta 1999, 402, 59-66.
27. Virtanen, V.; Bordin, G. Isoform separation of metallothioneins by capillary zone electrophoresis with Tris–tricine buffer in the presence or absence of methanol. Anal. Chem. A. 1998, 372, 231-239.
28. Guo, X.; Chan, H.M.; Guevremont, R.; Siu, K.W. Analysis of metallothioneins by means of capillary electrophoresis coupled to electrospray mass spectrometry with sheathless interfacing. Rapid Commun Mass Spectrom. 1999, 13, 500-507.
29. Tomalová, I.; Foltynová, P.; Kanický, V.; Preisler, J. MALDI MS and ICP MS detection of a single CE separation record: a tool for metalloproteomics. Anal Chem. 2014, 7, 647-654.
J.Met.Nano:
volume-2, issue-2
- Laboratory of Metallomics and Nanotechnologies – an initiator of the Metallomics Scientific Network formation
- Capillary electrophoresis of metallothionein
- Synthetic birnessites and buserites as heavy metal cation traps and environmental remedies
- Immunohistochemical detection of metallothionein
- MALDI-TOF MSI and electrochemical detection of metallothionein in chicken liver after cadmium exposure
- The use of MALDI MSI for the study of different tissues
- Utilization of graphene oxide electrophoretic deposition for construction of electrochemical sensors and biosensors
- Influence of Different Inducers on Ligninolytic Enzyme Activities
- Interaction of nanocarrier apoferritin with cytotoxic drug molecules
- Study of cell penetrating peptide and Europium(III) and Terbium(III) Schiff base complexes interaction
- HPV Detection in Leukocyte Samples of Spinocellular Carcinomas Using PCR
- Characterization of carbon quantum dots by capillary electrophoresis with laser-induced fluorescence detections
- Modification of anti-DNA antibodies with carbon quantum dots
- Fluorescence detection of carbon quantum dots assessed by stratospheric platform
 PDF
PDF