
Risks associated with a estrogen exposure to compounds with estrogenic activity and the possibility of elimination from aquatic environments
Zbynek Heger, Ondrej Zitka, Vojtech Adam, Rene Kizek
Estrogenic pollutants are a heterogeneous group of substances, contaminating the water environment. This group includes endogenous estrogens, naturally producing by organisms, exogenous estrogens that are contaminating the environment as a consequence of metabolical degradation and subsequent excretion and also other substances with estrogenic activity that have broad spectrum of industrial utilization. This work summarizes physiological molecular- -biological nature of action estrogenic substances, and the pathological effect on the organism, leading to plenty health complications, such as endocrine disruption or tumorigenesis. There are also discussed possible ways of degradation of these substances from waste water that are contaminated by substances with estrogenic potential.
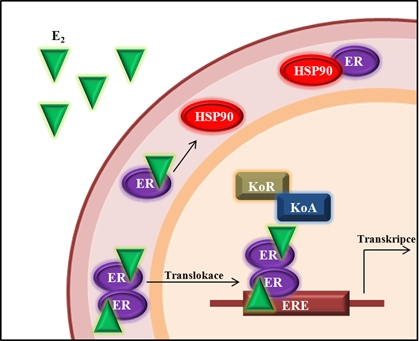 Fig. 1: Receptor (ER) after contact with a ligand (E2) is dissociated from the multiprotein complex with heat shock proteins (HSP 90). After creating homodimer occurs translocation to the nucleus and interact with koregulátory (repressors - KOR and coactivators - CoA) transcription. This leads to bind to estrogen responsivním element (ERE), which triggers transcription of mRNA. Subsequent translation provides for the formation of a new protein. Loosely redesigned eighth
Fig. 1: Receptor (ER) after contact with a ligand (E2) is dissociated from the multiprotein complex with heat shock proteins (HSP 90). After creating homodimer occurs translocation to the nucleus and interact with koregulátory (repressors - KOR and coactivators - CoA) transcription. This leads to bind to estrogen responsivním element (ERE), which triggers transcription of mRNA. Subsequent translation provides for the formation of a new protein. Loosely redesigned eighth
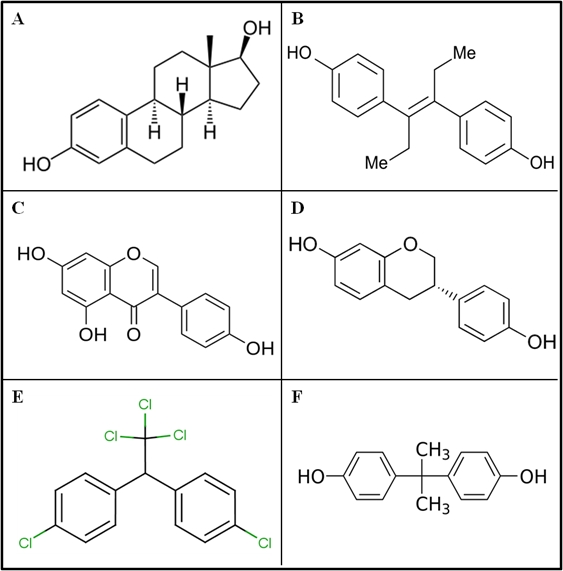 Fig. 2: Structural formulas of various substances with estrogenic activity. (A) 17β-estradiol (E2), belonging to the group of endogenous estrogens. (B) Diethylstilbestrol (DES) from the group of synthetic estrogens contained in contraceptives. (C) Genistein and (D) from the group of equol phytoestrogens. (E) 1,1,1-trichloro-2,2-bis (4-chlorophenyl) ethane (DDT), forbidden pesticide which still persists in the environment. (F) Bisphenol A (BPA) used in the production of polycarbonates.
Fig. 2: Structural formulas of various substances with estrogenic activity. (A) 17β-estradiol (E2), belonging to the group of endogenous estrogens. (B) Diethylstilbestrol (DES) from the group of synthetic estrogens contained in contraceptives. (C) Genistein and (D) from the group of equol phytoestrogens. (E) 1,1,1-trichloro-2,2-bis (4-chlorophenyl) ethane (DDT), forbidden pesticide which still persists in the environment. (F) Bisphenol A (BPA) used in the production of polycarbonates.
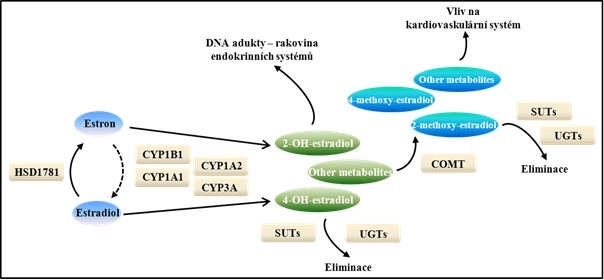 Fig. 3: Metabolic pathway biotransformation of estrone (E1) and estradiol (E2) with 17β-hydroxysteroid oxidoreductase (HSD1781). Metabolites resulting enzyme activity of cytochrome P450 families consisting of DNA adducts, further transformation by catechol-O-methyltransferase (COMT) originate mainly methoxy-estradiol, which can have both positive and negative influence on the cardiovascular system. Metabolites can be actively eliminated by sulphating (SUTSU) and glucuronidation enzymes (UGTs). Loosely revised according to 34th
Fig. 3: Metabolic pathway biotransformation of estrone (E1) and estradiol (E2) with 17β-hydroxysteroid oxidoreductase (HSD1781). Metabolites resulting enzyme activity of cytochrome P450 families consisting of DNA adducts, further transformation by catechol-O-methyltransferase (COMT) originate mainly methoxy-estradiol, which can have both positive and negative influence on the cardiovascular system. Metabolites can be actively eliminated by sulphating (SUTSU) and glucuronidation enzymes (UGTs). Loosely revised according to 34th
1. Crisp T. M., Clegg E. D., Cooper R. L., Anderson D. G., Baetcke K. P., Hoffmann J. L., Morrow M. S., Rodier D. J., Shaefer J. E., Touart L. W., Zeeman M. G., Patel Y. M., Wood W. P.: (1997).
2. Jeffries M. K. S., Conoan N. H., Cox M. B., Sangster J. L., Balsiger H. A., Bridges A. A., Cowman T., Knight L. A., Bartelt-Hunt S. L., Kolok A. S.: Aquatic Toxicology, 105, 189 (2011).
3. Tang X. J., Naveedullah, Hashmi M. Z., Zhang H., Qian M. R., Yu C. N., Shen C. F., Qin Z. H., Huang R. L., Qiao J. N., Chen Y. X.: Bulletin of Environmental Contamination and Toxicology, 90, 391 (2013).
4. Preda C., Ungureanu M. C., Vulpoi C.: Environmental Engineering and Management Journal, 11, 1697 (2012).
5. Qiang Z. M., Nie Y. F., Ben W. W., Qu J. H., Zhang H. Q.: Chemosphere, 91, 366 (2013).
6. DeRosa C., Richter P., Pohl H., Jones D. E.: Journal of Toxicology and Environmental Health-Part B-Critical Reviews, 1, 3 (1998).
7. Heldring N., Pike A., Andersson S., Matthews J., Cheng G., Hartman J., Tujague M., Strom A., Treuter E., Warner M., Gustafsson J. A.: Physiological Reviews, 87, 905 (2007).
8. Heger Z., Zitka O., Krizkova S., Beklova M., Kizek R., Adam V.: Neuroendocrinology Letters, 34, 123 (2013).
9. Acconcia F., Kumar R.: Cancer Letters, 238, 1 (2006).
10. Silva N., Peiris-John R., Wickremasinghe R., Senanayake H., Sathiakumar N.: Journal of Applied Toxicology, 32, 318 (2012).
11. Manavathi B., Dey O., Gajulapalli V. N. R., Bhatia R. S., Bugide S., Kumar R.: Endocrine Reviews, 34, 1 (2013).
12. Candelaria N. R., Liu K., Lin C. Y.: Journal of Cellular Biochemistry, 114, 2203 (2013).
13. O'Leary K. A., Jallow F., Rugowski D. E., Sullivan R., Sinkevicius K. W., Greene G. L., Schuler L. A.: Endocrinology, 154, 4483 (2013).
14. Abbondanza C., De Rosa C., D'Arcangelo A., Pacifico M., Spizuoco C., Piluso G., Di Zazzo E., Gazzerro P., Medici N., Moncharmont B., Puca G. A.: Journal of Cellular Physiology, 227, 964 (2012).
15. Losel R. M., Falkenstein E., Feuring M., Schultz A., Tillmann H. C., Rossol-Haseroth K., Wehling M.: Physiological Reviews, 83, 965 (2003).
16. Heger Z., Merlos Rodrigo M. A., Krizkova S., Zitka O., Beklova M., Kizek R., Adam V.: Oncology Letters, 7, 1341 (2014).
17. Mendes J. J. A.: Food and Chemical Toxicology, 40, 781 (2002).
18. Pereira M., Eppler E., Thorpe K. L., Wheeler J. R., Burkhardt-Holm P.: Environmental Toxicology, 29, 199 (2014).
19. Lange I. G., Hartel A., Meyer H. H. D.: Journal of Steroid Biochemistry and Molecular Biology, 83, 219 (2002).
20. Jin Y. X., Lin X. J., Miao W. Y., Wu T., Shen H. J., Chen S., Li Y. H., Pan Q. Q., Fu Z. W.: Toxicology Letters, 225, 392 (2014).
21. Laurent M., Antonio L., Sinnesael M., Dubois V., Gielen E., Classens F., Vanderschueren D.: Asian Journal of Andrology, 16, 213 (2014).
22. Billinghurst Z., Clare A. S., Fileman T., McEvoy J., Readman J., Depledge M. H.: Marine Pollution Bulletin, 36, 833 (1998).
23. Nicolas J. M.: Aquatic Toxicology, 45, 77 (1999).
24. Dang Z. C.: Environmental Pollution, 185, 266 (2014).
25. McRobb F. M., Sahagun V., Kufareva I., Abagyan R.: Environmental Science & Technology, 48, 1964 (2014).
26. Mann R. M., Hyne R. V., Choung C. B., Wilson S. P.: Environmental Pollution, 157, 2903 (2009).
27. Hung Y. C., Chang W. C., Chen L. M., Chang Y. Y., Wu L. Y., Chung W. M., Lin T. Y., Chen L. C., Ma W. L.: Journal of Cellular Physiology, 229, 752 (2014).
28. Etoh T., Nakai H.: Journal of Obstetrics and Gynaecology Research, 40, 820 (2014).
29. van Halteren H., Mulder D., Ruijter E.: Histopathology, 64, 787 (2014).
30. Vercellini P., Vigano P., Somigliana E., Fedele L.: Nature reviews. Endocrinology, 10, 261 (2014).
31. Athma P., Rappaport R., Swift M.: Cancer Genetics and Cytogenetics, 92, 130 (1996).
32. Leake R.: Lancet, 347, 1780 (1996).
33. Soule H. D., Vazquez J., Long A., Albert S., Brennan M.: Journal of the National Cancer Institute, 51, 1409 (1973).
34. Whirl-Carrillo M., McDonagh E. M., Hebert J. M., Gong L., Sangkuhl K., Thorn C. F., Altman R. B., Klein T. E.: Clinical Pharmacology & Therapeutics, 92, 414 (2012).
35. Yang L., Zahid M., Rogan E. G., Cavalieri E. L., Yager J. D., Visvanathan K., Groopman J., Davidson N. E., Kensler T. W.: Cancer Research, 73, (2013).
36. Cavalieri E., Rogan E.: Molecular Aspects of Medicine, 36, 1 (2014).
37. Carballa M., Omil F., Lema J. M., Llompart M., Garcia-Jares C., Rodriguez I., Gomez M., Ternes T.: Water Research, 38, 2918 (2004).
38. Press-Kristensen K., Ledin A., Schmidt J. E., Henze M.: Science of the Total Environment, 373, 122 (2007).
39. Pikaar I., Koelmans A. A., van Noort P. C. M.: Chemosphere, 65, 2343 (2006).
40. Snyder S. A., Westerhoff P., Yoon Y., Sedlak D. L.: Environmental Engineering Science, 20, 449 (2003).
41. Lai K. M., Johnson K. L., Scrimshaw M. D., Lester J. N.: Environmental Science & Technology, 34, 3890 (2000).
42. Chang H. S., Choo K. H., Lee B., Choi S. J.: Journal of Hazardous Materials, 172, 1 (2009).
43. Pyrzynska K., Stafiej A., Biesaga M.: Microchimica Acta, 159, 293 (2007).
44. Tsutsumi Y., Haneda T., Nishida T.: Chemosphere, 42, 271 (2001).
45. Gabriel F. L. P., Heidlberger A., Rentsch D., Giger W., Guenther K., Kohler H. P. E.: Journal of Biological Chemistry, 280, 15526 (2005).
46. Sasaki M., Maki J., Oshiman K., Matsumura Y., Tsuchido T.: Biodegradation, 16, 449 (2005).
47. Sethunathan N., Megharaj M., Chen Z. L., Williams B. D., Lewis G., Naidu R.: Journal of Agricultural and Food Chemistry, 52, 3030 (2004).
48. Robinson B. J., Hellou J.: Science of the Total Environment, 407, 5713 (2009).
J.Met.Nano:
volume-1, issue-2
- Nanopores as a modern tool for DNA sequencing
- Use of mass spectrometry technique (MALDI-TOF/TOF) for the characterization of metallothionein in biological systems
- Doxorubicin: help and threat in cancer therapy
- Therapeutical application of antiviral peptides against influenza virus
- Effect of antimicrobial peptides
- Magnetic micro and nanoparticles for unique sequences barcoding
- Study of interaction of the receptor for the hemagglutinin
- Risks associated with exposure to estrogens and substances with estrogenic activity and their elimination from water environment
- Electrochemical study of flavonoids in wine
- Electrochemical analysis of resveratrol in wine
- Optimization of multiplex PCR
- Identification of microorganisms using MALDI–TOF MS
- Influence study of non-platinum cytotoxic drugs on polymerase chain reaction
 PDF
PDF