
Nanopores as a modern tool for DNA sequencing
Jiri Kudr, Branislav Ruttkay-Nedecky, Vojtech Adam, Rene Kizek
A nanopore-based devices are extremely sensitive analytical techniques, which uses the electrophoretic translocation of molecules in solution through a nano-scale pores. The nanopores, which mimic the functions of natural ion channels, seems to be the promissing tool for future fast and low-cost DNA sequencing. However, some difficulties in generating usable sequence data have to be solved. In this article the nanopores were reviewed. In the first part the development of nanopore technique was described and the ubiquitous presence of nanopores in living cells was highlighted. Next, the most important part of the principles of nanopore analysis was described, and the knowledges about biological and solid-state nanopores were summarized. Also the pros and cons of both kinds of nanopores and different approaches designed to circumvent the issues were mentioned.
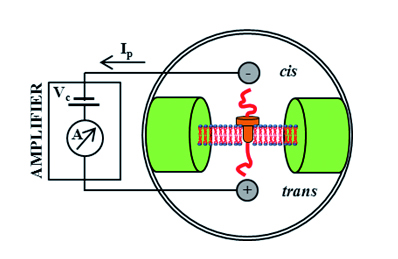 Fig. 1: Illustrative schematic of the apparatus used for the analysis of nucleic acids (A). Amplifier applied voltage Vc and detects changes in the current passing through hemolysin Ip. When applying a voltage of 120 mV using 1 M KCl as electrolyte passes hemolysin reference current of 120 pA, which is the translocation of ssDNA reduced to 15 Pa (B).
Fig. 1: Illustrative schematic of the apparatus used for the analysis of nucleic acids (A). Amplifier applied voltage Vc and detects changes in the current passing through hemolysin Ip. When applying a voltage of 120 mV using 1 M KCl as electrolyte passes hemolysin reference current of 120 pA, which is the translocation of ssDNA reduced to 15 Pa (B).
1. Graham M. D.: Cytometry Part A, 83, 1057 (2013).
2. Deblois R. W., Bean C. P., Wesley R. K. A.: Journal of Colloid and Interface Science, 61, 323 (1977).
3. Wanunu M.: Physics of Life Reviews, 9, 125 (2012).
4. Sanguinetti M. C., Tristani-Firouzi M.: Nature, 440, 463 (2006).
5. Gorlich D., Kutay U.: Annual Review of Cell and Developmental Biology, 15, 607 (1999).
6. Guasch A., Pous J., Ibarra B., Gomis-Ruth F. X., Valpuesta J. M., Sousa N., Carrascosa J. L., Coll M.: Journal of Molecular Biology, 315, 663 (2002).
7. Diep B. A., Otto M.: Trends in Microbiology, 16, 361 (2008).
8. Branton D., Deamer D. W., Marziali A., Bayley H., Benner S. A., Butler T., Di Ventra M., Garaj S., Hibbs A., Huang X. H., Jovanovich S. B., Krstic P. S., Lindsay S., Ling X. S. S., Mastrangelo C. H., Meller A., Oliver J. S., Pershin Y. V., Ramsey J. M., Riehn R., Soni G. V., Tabard-Cossa V., Wanunu M., Wiggin M., Schloss J. A.: Nature Biotechnology, 26, 1146 (2008).
9. Baldarelli R., Branton D., Church G., Deamer D. W., Kasianowicz J.: (1998).
10. van Hooft J. A., Wadman W. J.: Journal of Neurophysiology, 89, 1864 (2003).
11. Purcell E. K., Liu L. Q., Thomas P. V., Duncan R. K.: Plos One, 6, (2011).
12. Laszlo A. H., Derrington I. M., Brinkerhoff H., Langford K. W., Nova I. C., Samson J. M., Bartlett J. J., Pavlenok M., Gundlach J. H.: Proceedings of the National Academy of Sciences of the United States of America, 110, 18904 (2013).
13. Meller A., Nivon L., Brandin E., Golovchenko J., Branton D.: Proceedings of the National Academy of Sciences of the United States of America, 97, 1079 (2000).
14. Storm A. J., Chen J. H., Zandbergen H. W., Dekker C.: Physical Review E, 71, (2005).
15. Venkatesan B. M., Bashir R.: Nature Nanotechnology, 6, 615 (2011).
16. Walker B., Krishnasastry M., Zorn L., Kasianowicz J., Bayley H.: Journal of Biological Chemistry, 267, 10902 (1992).
17. Walker B., Kasianowicz J., Krishnasastry M., Bayley H.: Protein Engineering, 7, 655 (1994).
18. Kasianowicz J. J., Brandin E., Branton D., Deamer D. W.: Proceedings of the National Academy of Sciences of the United States of America, 93, 13770 (1996).
19. Song L. Z., Hobaugh M. R., Shustak C., Cheley S., Bayley H., Gouaux J. E.: Science, 274, 1859 (1996).
20. Akeson M., Branton D., Kasianowicz J. J., Brandin E., Deamer D. W.: Biophysical Journal, 77, 3227 (1999).
21. Meller A., Branton D.: Electrophoresis, 23, 2583 (2002).
22. Clamer M., Hofler L., Mikhailova E., Viero G., Bayley H.: Acs Nano, 8, 1364 (2014).
23. Lee J. S.: Protein and Peptide Letters, 21, 247 (2014).
24. Wang G. H., Wang L., Han Y. J., Zhou S., Guan X. Y.: Biosensors & Bioelectronics, 53, 453 (2014).
25. Asandei A., Schiopu I., Iftemi S., Mereuta L., Luchian T.: Langmuir, 29, 15634 (2013).
26. Stahl C., Kubetzko S., Kaps I., Seeber S., Engelhardt H., Niederweis M.: Molecular Microbiology, 40, 451 (2001).
27. Faller M., Niederweis M., Schulz G. E.: Science, 303, 1189 (2004).
28. Butler T. Z., Pavlenok M., Derrington I. M., Niederweis M., Gundlach J. H.: Proceedings of the National Academy of Sciences of the United States of America, 105, 20647 (2008).
29. Derrington I. M., Butler T. Z., Collins M. D., Manrao E., Pavlenok M., Niederweis M., Gundlach J. H.: Proceedings of the National Academy of Sciences of the United States of America, 107, 16060 (2010).
30. Fennouri A., Przybylski C., Pastoriza-Gallego M., Bacri L., Auvray L., Daniel R.: Acs Nano, 6, 9672 (2012).
31. Soskine M., Biesemans A., Moeyaert B., Cheley S., Bayley H., Maglia G.: Nano Letters, 12, 4895 (2012).
32. Mohammad M. M., Iyer R., Howard K. R., McPike M. P., Borer P. N., Movileanu L.: Journal of the American Chemical Society, 134, 9521 (2012).
33. Chen Y. S., Lee C. H., Hung M. Y., Pan H. A., Chiou J. C., Huang G. S.: Nature Nanotechnology, 8, 452 (2013).
34. Wang H. Y., Li Y., Qin L. X., Heyman A., Shoseyov O., Willner I., Long Y. T., Tian H.: Chemical Communications, 49, 1741 (2013).
35. Stoddart D., Heron A. J., Mikhailova E., Maglia G., Bayley H.: Proceedings of the National Academy of Sciences of the United States of America, 106, 7702 (2009).
36. Deamer D. W., Branton D.: Accounts of Chemical Research, 35, 817 (2002).
37. Kawano R., Schibel A. E. P., Cauley C., White H. S.: Langmuir, 25, 1233 (2009).
38. de Zoysa R. S. S., Jayawardhana D. A., Zhao Q. T., Wang D. Q., Armstrong D. W., Guan X. Y.: Journal of Physical Chemistry B, 113, 13332 (2009).
39. Rincon-Restrepo M., Milthallova E., Bayley H., Maglia G.: Nano Letters, 11, 746 (2011).
40. Hurt N., Wang H. Y., Akeson M., Lieberman K. R.: Journal of the American Chemical Society, 131, 3772 (2009).
41. Wu H. C., Astier Y., Maglia G., Mikhailova E., Bayley H.: Journal of the American Chemical Society, 129, 16142 (2007).
42. Lieberman K. R., Cherf G. M., Doody M. J., Olasagasti F., Kolodji Y., Akeson M.: Journal of the American Chemical Society, 132, 17961 (2010).
43. Gong X. Q., Patil A. V., Ivanov A. P., Kong Q. Y., Gibb T., Dogan F., deMello A. J., Edel J. B.: Analytical Chemistry, 86, 835 (2014).
44. Hu G. H., Mao M., Ghosal S.: Nanotechnology, 23, (2012).
45. Tabard-Cossa V., Trivedi D., Wiggin M., Jetha N. N., Marziali A.: Nanotechnology, 18, (2007).
46. Storm A. J., Chen J. H., Ling X. S., Zandbergen H. W., Dekker C.: Nature Materials, 2, 537 (2003).
47. Hoogerheide D. P., Garaj S., Golovchenko J. A.: Physical Review Letters, 105, (2010).
48. Smeets R. M. M., Keyser U. F., Wu M. Y., Dekker N. H., Dekker C.: Physical Review Letters, 97, (2006).
49. Kim W. K., Nam W. H., Kim S. H., Rhee S. W.: Journal of Chemical Engineering of Japan, 38, 578 (2005).
50. Li J., Stein D., McMullan C., Branton D., Aziz M. J., Golovchenko J. A.: Nature, 412, 166 (2001).
51. Fischbein M. D., Drndic M.: Applied Physics Letters, 93, (2008).
52. Merchant C. A., Healy K., Wanunu M., Ray V., Peterman N., Bartel J., Fischbein M. D., Venta K., Luo Z. T., Johnson A. T. C., Drndic M.: Nano Letters, 10, 2915 (2010).
53. Postma H. W. C.: Nano Letters, 10, 420 (2010).
54. Venkatesan B. M., Shah A. B., Zuo J. M., Bashir R.: Advanced Functional Materials, 20, 1266 (2010).
55. Liu H. T., He J., Tang J. Y., Liu H., Pang P., Cao D., Krstic P., Joseph S., Lindsay S., Nuckolls C.: Science, 327, 64 (2010).
56. Jin X. Z., Aluru N. R.: Microfluidics and Nanofluidics, 11, 297 (2011).
57. Karnik R., Duan C. H., Castelino K., Daiguji H., Majumdar A.: Nano Letters, 7, 547 (2007).
J.Met.Nano:
volume-1, issue-2
- Nanopores as a modern tool for DNA sequencing
- Use of mass spectrometry technique (MALDI-TOF/TOF) for the characterization of metallothionein in biological systems
- Doxorubicin: help and threat in cancer therapy
- Therapeutical application of antiviral peptides against influenza virus
- Effect of antimicrobial peptides
- Magnetic micro and nanoparticles for unique sequences barcoding
- Study of interaction of the receptor for the hemagglutinin
- Risks associated with exposure to estrogens and substances with estrogenic activity and their elimination from water environment
- Electrochemical study of flavonoids in wine
- Electrochemical analysis of resveratrol in wine
- Optimization of multiplex PCR
- Identification of microorganisms using MALDI–TOF MS
- Influence study of non-platinum cytotoxic drugs on polymerase chain reaction
 PDF
PDF