
Use of mass spectrometry technique (MALDI-TOF/TOF) for the characterization of metallothionein in biological systems
Miguel Angel Merlos Rodrigo, Ondrej Zitka, Vojtech Adam, Rene Kizek
Metallothioneins (MTs) were discovered in 1957 and identified as low-molecular weight sulfhydryl-rich proteins. MTs belong to a superfamily of intracellular metal-binding proteins, present in virtually all living organisms, with features common to the archetypal. MT was first isolated from horse kidney and characterized by Margoshes and Vallee [1]. In this work, we wish to briefly summarize the current knowledge regarding the MT forms. All vertebrates examined contain two or more distinct MT isoforms designated MT-1 through MT-4. The three-dimensional structures of MTs from mammalian that have been determined so far show a monomeric protein composed of two globular domains, each encompassing a metal–thiolate cluster. The metallothionein isoform A (MTA) is a 64-residue metalloprotein, which contains essentially the same number of metal-chelating Cys–Cys and Cys–Xxx–Cys motifs (where Xxx stands for any amino acid, other than Cys) and metal ions [2, 3]. These cysteine-rich proteins are localized in cytoplasm and some organelles, predominantly in mitochondria, where their presence is sensitively and strictly regulated by the oxidative state induced by mitochondrial respiration. Depending on the cell state, but especially presence of oxidative stress, MTs are rapidly translocated to the nucleus through nuclear pore complexes. MT localized in the nuclei is oxidized there and it is transported to cytosol; this system is balanced [3].
 Figure.1 Photos of 3-dimensional structure of MT isolated from liver rabbit liver without (A) and with heavy metal (B). These photos were created by an advanced molecule editor (Avogadro 1.1.1) in our laboratory.
Figure.1 Photos of 3-dimensional structure of MT isolated from liver rabbit liver without (A) and with heavy metal (B). These photos were created by an advanced molecule editor (Avogadro 1.1.1) in our laboratory.
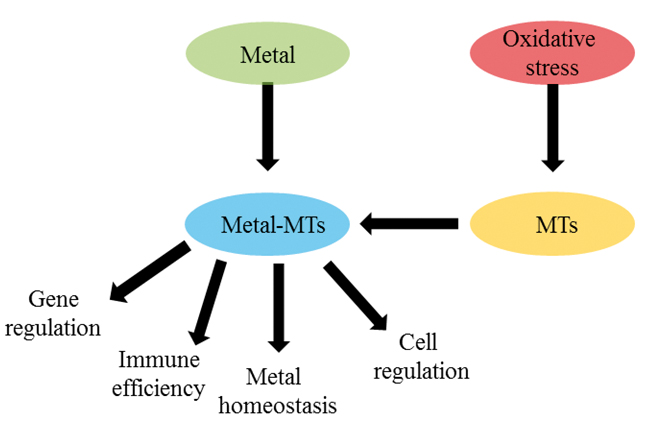 Figure 2. Overview of MT function
Figure 2. Overview of MT function
1. Tan, Y., et al., Metallothionein expression and nuclear size-in benign, borderline, and malignant serous ovarian tumours. Journal of Pathology, 1999. 189(1): p. 60-65.
2. Vasak, M. and D.W. Hasler, Metallothioneins: new functional and structural insights. Current Opinion in Chemical Biology, 2000. 4(2): p. 177-183.
3. Babula, P., et al., Mammalian metallothioneins: properties and functions. Metallomics, 2012. 4(8): p. 739-750.
4. Kagi, J.H.R. and M. Vasak, CHEMISTRY AND BIOCHEMISTRY OF METALLOTHIONEIN. Highlights of Modern Biochemistry, Vols 1-2, ed. A. Kotyk, et al.1989, Zeist: Vsp Bv. 299-308.
5. Chen, P., et al., Stoichiometry and cluster specificity of copper binding to metallothionein: Homogeneous metal clusters. Biochemical Journal, 1996. 317: p. 395-402.
6. Coyle, P., et al., Metallothionein: The multipurpose protein. Cellular and Molecular Life Sciences, 2002. 59(4): p. 627-647.
7. Thirumoorthy, N., et al., A Review of Metallothionein Isoforms and their Role in Pathophysiology. World Journal of Surgical Oncology, 2011. 9.
8. Trnkova, L., et al., Paramagnetic antibody-modified microparticles coupled with voltammetry as a tool for isolation and detection of metallothionen as a bioindicator of metal pollution. Journal of Environmental Monitoring, 2011. 13(10): p. 2763-2769.
9. Cai, L., et al., Metallothionein in radiation exposure: its induction and protective role. Toxicology, 1999. 132(2-3): p. 85-98.
10. Ruttkay-Nedecky, B., et al., The Role of Metallothionein in Oxidative Stress. International Journal of Molecular Sciences, 2013. 14(3): p. 6044-6066.
11. Carpene, E., G. Andream, and G. Isam, Metallothionein functions and structural characteristics. Journal of Trace Elements in Medicine and Biology, 2007. 21: p. 35-39.
12. Gehrig, P.M., et al., Electrospray ionization mass spectrometry of zinc, cadmium, and copper metallothioneins: Evidence for metal-binding cooperativity. Protein Science, 2000. 9(2): p. 395-402.
13. Bizon, A., et al., Changes in pro/antioxidant balance in smoking and non-smoking pregnant women with intrauterine growth restriction. Reproductive Toxicology, 2011. 32(3): p. 360-367.
14. Cai, L., J.B. Klein, and Y.J. Kang, Metallothionein inhibits peroxynitrite-induced DNA and lipoprotein damage. Journal of Biological Chemistry, 2000. 275(50): p. 38957-38960.
15. Chiaverini, N. and M. De Ley, Protective effect of metallothionein on oxidative stress-induced DNA damage. Free Radical Research, 2010. 44(6): p. 605-613.
16. Langmade, S.J., et al., The transcription factor MTF-1 mediates metal regulation of the mouse ZnT1 gene. Journal of Biological Chemistry, 2000. 275(44): p. 34803-34809.
17. Jacob, C., W. Maret, and B.L. Vallee, Control of zinc transfer between thionein, metallothionein, and zinc proteins. Proceedings of the National Academy of Sciences of the United States of America, 1998. 95(7): p. 3489-3494.
18. Andrews, G.K., Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochemical Pharmacology, 2000. 59(1): p. 95-104.
19. Giedroc, D.P., X.H. Chen, and J.L. Apuy, Metal response element (MRE)-binding transcription factor-1 (MTF-1): Structure, function, and regulation. Antioxidants & Redox Signaling, 2001. 3(4): p. 577-596.
20. Thirumoorthy, N., et al., Metallothionein: An overview. World Journal of Gastroenterology, 2007. 13(7): p. 993-996.
21. Takahashi, S., Molecular functions of metallothionein and its role in hematological malignancies. Journal of Hematology & Oncology, 2012. 5.
22. Zhang, G.X., et al., A comparative study on interactions of cisplatin and ruthenium arene anticancer complexes with metallothionein using MALDI-TOF-MS. International Journal of Mass Spectrometry, 2011. 307(1-3): p. 79-84.
23. Zamirska, A., et al., Expression of Metallothioneins in Cutaneous Squamous Cell Carcinoma and Actinic Keratosis. Pathology & Oncology Research, 2012. 18(4): p. 849-855.
24. Ohshio, G., et al., Immunohistochemical study of metallothionein in pancreatic carcinomas. Journal of Cancer Research and Clinical Oncology, 1996. 122(6): p. 351-355.
25. Cardoso, S.V., et al., Expression of Metallothionein and p53 Antigens are Correlated in Oral Squamous Cell Carcinoma. Anticancer Research, 2009. 29(4): p. 1189-1193.
26. Joseph, M.G., et al., Metallothionein expression in patients with small cell carcinoma of the lung - Correlation with other molecular markers and clinical outcome. Cancer, 2001. 92(4): p. 836-842.
27. Peng, B., et al., Microarray-Assisted Pathway Analysis Identifies MT1X & NF kappa B as Mediators of TCRP1-Associated Resistance to Cisplatin in Oral Squamous Cell Carcinoma. Plos One, 2012. 7(12).
28. Mita, M., et al., Metallothionein deficiency exacerbates chronic inflammation associated with carcinogenesis in stomach of mice infected with Helicobacter pylori. Journal of Toxicological Sciences, 2012. 37(6): p. 1261-1265.
29. Ozer, H., et al., Immunohistochemistry with apoptotic-antiapoptotic proteins (p53, p21, bax, bcl-2), c-kit, telomerase, and metallothionein as a diagnostic aid in benign, borderline, and malignant serous and mucinous ovarian tumors. Diagnostic Pathology, 2012. 7.
30. Duval, D., et al., Apoptosis and differentiation commitment: novel insights revealed by gene profiling studies in mouse embryonic stem cells. Cell Death and Differentiation, 2006. 13(4): p. 564-575.
31. Bagheri, P.M., et al., Differential quantitative zinc-induced expression of human metallothionein isogenes in haematopoietic precursor cell lines. Journal of Trace Elements in Medicine and Biology, 2009. 23(2): p. 124-131.
32. Abdel-Mageed, A.B., et al., Erythropoietin-induced metallothionein gene expression: Role in proliferation of K562 cells. Experimental Biology and Medicine, 2003. 228(9): p. 1033-1039.
33. Cherian, M.G., A. Jayasurya, and B.H. Bay, Metallothioneins in human tumors and potential roles in carcinogenesis. Mutation Research-Fundamental and Molecular Mechanisms of Mutagenesis, 2003. 533(1-2): p. 201-209.
34. Jin, R.X., et al., Clinicopathological significance of metallothioneins in breast cancer. Pathology & Oncology Research, 2004. 10(2): p. 74-79.
35. Werynska, B., et al., Metallothionein IF and 2A overexpression predicts poor outcome of non-small cell lung cancer patients. Experimental and Molecular Pathology, 2013. 94(1): p. 301-308.
36. Peixoto-Santos, J.E., et al., Increased Metallothionein I/II Expression in Patients with Temporal Lobe Epilepsy. Plos One, 2012. 7(9).
37. Jakovac, H., et al., Time-course expression of metallothioneins and tissue metals in chronic relapsing form of experimental autoimmune encephalomyelitis. Histology and Histopathology, 2011. 26(2): p. 233-245.
38. Cho, Y.T., et al., Matrix-assisted laser desorption ionization/time-of-flight mass spectrometry for clinical diagnosis. Clinica Chimica Acta, 2013. 415: p. 266-275.
39. Merlos, M., et al., MALDI-TOF MS as evolving cancer diagnostic tool: A review. Journal of Pharmaceutical and Biomedical Analysis, 2014. Volume 95 p. Pages 245–255.
40. Andon, B., J. Barbosa, and V. Sanz-Nebot, Separation and characterization of rabbit liver apothioneins by capillary electrophoresis coupled to electrospray ionization time-of-flight mass spectrometry. Electrophoresis, 2006. 27(18): p. 3661-3670.
41. Zhao, R., et al., Separation of rabbit liver metallothionein sub-isoforms by RP-HPLC with MALDI-TOF-MS detection. Chemical Journal of Chinese Universities-Chinese, 2002. 23(6): p. 1086-1090.
42. Chen, C.Y., et al., Proteomic analysis on multi-drug resistant cells HL-60/DOX of acute myeloblastic leukemia (vol 48, pg 115, 2005). Chinese Journal of Physiology, 2005. 48(4): p. 230-230.
43. Zhang, Y., et al., On-plate enrichment methods for MALDI-MS analysis in proteomics. Analytical Methods, 2012. 4(9): p. 2622-2631
.
44. Wang, R.Y., et al., Simple method for identification of metallothionein isoforms in cultured human prostate cells by MALDI-TOF/TOF mass spectrometry. Analytical Chemistry, 2007. 79(12): p. 4433-4441.
45. Zitka, O., et al., Matrix Metalloproteinases. Current Medicinal Chemistry, 2010. 17(31): p. 3751-3768.
46. Lo, Y.C., et al., Terpyridine Platinum(II) Complexes Inhibit Cysteine Proteases by Binding to Active-site Cysteine. Journal of Biomolecular Structure & Dynamics, 2011. 29(2): p. 267-282.
47. Zhang, G., et al., A comparative study on interactions of cisplatin and ruthenium arene anticancer complexes with metallothionein using MALDI-TOF-MS. International Journal of Mass Spectrometry, 2011. 307(1-3): p. 79-84.
48. Smirnov, I.P., et al., Suppression of alpha-cyano-4-hydroxycinnamic acid matrix clusters and reduction of chemical noise in MALDI-TOF mass spectrometry. Analytical Chemistry, 2004. 76(10): p. 2958-2965.
J.Met.Nano:
volume-1, issue-2
- Nanopores as a modern tool for DNA sequencing
- Use of mass spectrometry technique (MALDI-TOF/TOF) for the characterization of metallothionein in biological systems
- Doxorubicin: help and threat in cancer therapy
- Therapeutical application of antiviral peptides against influenza virus
- Effect of antimicrobial peptides
- Magnetic micro and nanoparticles for unique sequences barcoding
- Study of interaction of the receptor for the hemagglutinin
- Risks associated with exposure to estrogens and substances with estrogenic activity and their elimination from water environment
- Electrochemical study of flavonoids in wine
- Electrochemical analysis of resveratrol in wine
- Optimization of multiplex PCR
- Identification of microorganisms using MALDI–TOF MS
- Influence study of non-platinum cytotoxic drugs on polymerase chain reaction
 PDF
PDF