
Study of interaction of the receptor for the hemagglutinin
Petr Michalek, Ondrej Zitka, Rene Kizek
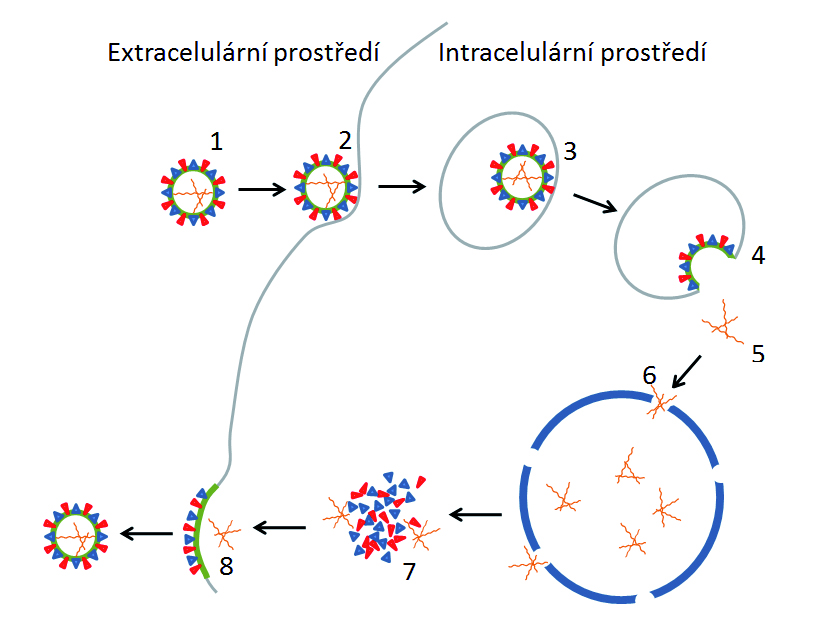
Influenza viruses compared to other viruses are characterized by considerable genetic variability. From the view of the ability of the virus to infect the host cell the determining factor is the structure of the surface antigens of the virus and the host cell receptor structure. To change the preferential binding of the virus to various cell receptors just one amino acid substitution in the primary structure of hemagglutinin is enough. The cause of this change in the antigenic properties is either the antigenic shift or antigenic shift. Thereby, new influenza strains with new antigenic type can develop very quickly and a human population is not capable to immunologically distinguished it with sufficient speed. Yet widely used drugs are increasingly perceived as controversial, due to their declining benefits and vice versa to growing negative effects. And despite considerable progress in the research of influenza viruses and their receptors at the molecular and structural level no sufficiently effective alternative has not been introduced yet.
1. Johnson N., Mueller J.: Bulletin of the History of Medicine, 76, 105 (2002).
2. Ginsberg J., Mohebbi M. H., Patel R. S., Brammer L., Smolinski M. S., Brilliant L.: Nature, 457, 1012 (2009).
3. Basler C. F., Aguilar P. V.: Antiviral Research, 79, 166 (2008).
4. Wiley D. C., Skehel J. J.: Annual Review of Biochemistry, 56, 365 (1987).
5. Russell R. J., Kerry P. S., Stevens D. J., Steinhauer D. A., Martin S. R., Gamblin S. J., Skehel J. J.: Proceedings of the National Academy of Sciences of the United States of America, 105, 17736 (2008).
6. Gamblin S. J., Haire L. F., Russell R. J., Stevens D. J., Xiao B., Ha Y., Vasisht N., Steinhauer D. A., Daniels R. S., Elliot A., Wiley D. C., Skehel J. J.: Science, 303, 1838 (2004).
7. Madhusoodanan M., Lazaridis T.: Biophysical Journal, 84, 1926 (2003).
8. Skehel J. J., Wiley D. C.: Annual Review of Biochemistry, 69, 531 (2000).
9. Stevens J., Blixt O., Tumpey T. M., Taubenberger J. K., Paulson J. C., Wilson I. A.: Science, 312, 404 (2006).
10. Isin B., Doruker P., Bahar I.: Biophysical Journal, 82, 569 (2002).
11. Garten W., Klenk H. D.: Trends in Microbiology, 7, 99 (1999).
12. Gamblin S. J., Skehel J. J.: Journal of Biological Chemistry, 285, 28403 (2010).
13. Bullough P. A., Hughson F. M., Skehel J. J., Wiley D. C.: Nature, 371, 37 (1994).
14. Walker J. A., Molloy S. S., Thomas G., Sakaguchi T., Yoshida T., Chambers T. M., Kawaoka Y.: Journal of Virology, 68, 1213 (1994).
15. van Riel D., Munster V. J., de Wit E., Rimmelzwaan G. F., Fouchier R. A. M., Osterhaus A., Kuiken T.: Science, 312, 399 (2006).
16. Brown I. H.: Options for the Control of Influenza Iv, 1219, 173 (2001).
17. Matrosovich M., Tuzikov A., Bovin N., Gambaryan A., Klimov A., Castrucci M. R., Donatelli I., Kawaoka Y.: Journal of Virology, 74, 8502 (2000).
18. Glaser L., Stevens J., Zamarin D., Wilson I. A., Garcia-Sastre A., Tumpey T. M., Basler C. F., Taubenberger J. K., Palese P.: Journal of Virology, 79, 11533 (2005).
19. Rogers G. N., Paulson J. C., Daniels R. S., Skehel J. J., Wilson I. A., Wiley D. C.: Nature, 304, 76 (1983).
20. Chen J. Z., Deng Y. M.: Virology Journal, 6, (2009).
21. Carrat F., Flahault A.: Vaccine, 25, 6852 (2007).
22. Hamilton B. S., Whittaker G. R., Daniel S.: Viruses-Basel, 4, 1144 (2012).
23. Ilyushina N. A., Govorkova E. A., Webster R. G.: Virology, 341, 102 (2005).
24. Lackenby A., Thompson C. I., Democratis J.: Current Opinion in Infectious Diseases, 21, 626 (2008).
25. Torres J., et al.: Bioinformation, 8, 870 (2012).
26. Yang J., Li M. M., Shen X. T., Liu S. W.: Viruses-Basel, 5, 352 (2013).
27. Lopez-Martinez R., Ramirez-Salinas G. L., Correa-Basurto J., Barron B. L.: Plos One, 8, (2013).
28. Jones J. C., Turpin E. A., Bultmann H., Brandt C. R., Schultz-Cherry S.: Journal of Virology, 80, 11960 (2006).
29. Beppu Y., Imamura Y., Tashiro M., Towatari T., Ariga H., Kido H.: Journal of Biochemistry, 121, 309 (1997).
30. Zhirnov O. P., Ovcharenko A. V., Bukrinskaya A. G.: Journal of General Virology, 65, 191 (1984).
31. Kido H., Yokogoshi Y., Sakai K., Tashiro M., Kishino Y., Fukutomi A., Katunuma N.: Journal of Biological Chemistry, 267, 13573 (1992).
32. Kido H., Sakai K., Kishino Y., Tashiro M.: Febs Letters, 322, 115 (1993).
33. Bottcher-Friebertshauser E., Stein D. A., Klenk H. D., Garten W.: Journal of Virology, 85, 1554 (2011).
34. Lupfer C., Stein D. A., Mourich D. V., Tepper S. E., Iversen P. L., Pastey M.: Archives of Virology, 153, 929 (2008).
35. Gabriel G., Nordmann A., Stein D. A., Iversen P. L., Klenk H. D.: Journal of General Virology, 89, 939 (2008).
J.Met.Nano:
volume-1, issue-2
- Nanopores as a modern tool for DNA sequencing
- Use of mass spectrometry technique (MALDI-TOF/TOF) for the characterization of metallothionein in biological systems
- Doxorubicin: help and threat in cancer therapy
- Therapeutical application of antiviral peptides against influenza virus
- Effect of antimicrobial peptides
- Magnetic micro and nanoparticles for unique sequences barcoding
- Study of interaction of the receptor for the hemagglutinin
- Risks associated with exposure to estrogens and substances with estrogenic activity and their elimination from water environment
- Electrochemical study of flavonoids in wine
- Electrochemical analysis of resveratrol in wine
- Optimization of multiplex PCR
- Identification of microorganisms using MALDI–TOF MS
- Influence study of non-platinum cytotoxic drugs on polymerase chain reaction
 PDF
PDF