
The role of phytochelatins in plant and animals: A review
Miguel Angel Merlos, Petr Michálek, Olga Kryštofová, Ondřej Zítka, Vojtěch Adam, René Kizeka
Increasing emissions of heavy metals such as cadmium, mercury, and arsenic into the environment pose an acute problem for all organisms. As a mass of protection, many of them, develop mechanisms of full resistance or at least exhibit partially resisting toward these effects. In this way, based on the chemical similarity of the involved metallic species, they are able, to replace them with viable metals necessary for the effective functioning of the cell. These heavy metals may be bound to the functional groups of proteins and modify their structure and through this also affect their physiological function 1, 2. Higher plants, algae, certain yeasts and animals are able to respond to heavy metals by synthesizing phytochelatins (PCs) and related cysteine-rich polypeptides. Phytochelatin synthases are γ-glutamylcysteine (γ-Glu-Cys) dipeptidyl transpeptidases that catalyze the synthesis of heavy metal-binding PCs 3, 4. PCs, cysteine-rich peptides, are produced from glutamine, cysteine and glycine. Unlike commonmetal-binding structures, MT and GSH, PCs are not gene-encoded, but enzymatically synthesized peptides 5. PCs have been identified in a wide variety of plant species, microorganisms and some invertebrates 6-10. They are structurally related to glutathione (GSH) and were presumed to be the products of a biosynthetic pathway. Numerous physiological, biochemical and genetic studies have confirmed GSH as the substrate for PCs biosynthesis 11, 12. The general structure of PCs is (c-Glu-Cys)n-Gly, with increasing repetitions of the dipeptide Glu-Cys linked through a c-carboxylamide bond (Fig 1), where n varies from 2 to 11, but typically reaching not further than five 13. Except glycine, also other amino acid residues can be found on C-terminal end of (γ-Glu-Cys)n peptides. Examples of which, like Ser, Glu, Gln and Ala are often found at this position in some plant species, and they are assumed to be functionally analogous and synthesised via essentially similar biochemical pathways 14, 15. In in vitro studies of PC synthase expressed in E. coli or in S. cerevisiae, the enzyme was activated to varying extents by Cd, Cu, Ag, Hg, Zn and Pb ions 16-18. PC synthase genes were also isolated in A.thaliana 16 and T.aestivum 18. Genes homologous to those from A.thaliana and T.aestivum were also found in S.pombe and C.elegans, suggesting the existence of PC synthase genes in more species 19.
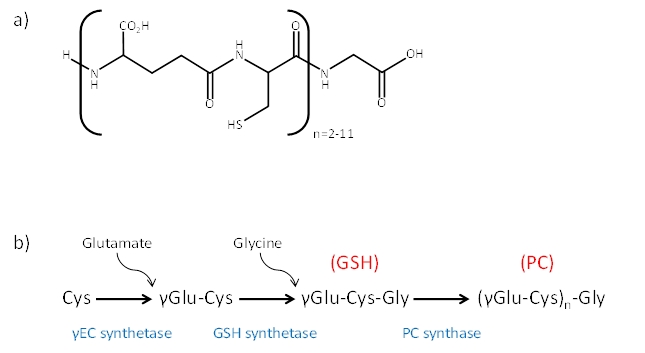
Figure 1. a) The general structure of PCs. b) Biosynthesis of phytochelatins.
1. Sharma, S.S. and K.-J. Dietz, The relationship between metal toxicity and cellular redox imbalance. Trends in Plant Science, 2009. 14(1): p. 43-50.
2. Yadav, S.K., Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. South African Journal of Botany, 2010. 76(2): p. 167-179.
3. Vatamaniuk, O.K., et al., Mechanism of heavy metal ion activation of phytochelatin (PC) synthase - Blocked thiols are sufficient for PC synthase-catalyzed transpeptidation of glutathione and related thiol peptides. Journal of Biological Chemistry, 2000. 275(40): p. 31451-31459.
4. Rea, P.A., Phytochelatin synthase: of a protease a peptide polymerase made. Physiologia Plantarum, 2012. 145(1): p. 154-164.
5. Noctor, G. and C.H. Foyer, Ascorbate and glutathione: Keeping active oxygen under control, in Annual Review of Plant Physiology and Plant Molecular Biology, R.L. Jones, C.R. Somerville, and V. Walbot, Editors. 1998, Annual Reviews Inc. {a}, P.O. Box 10139, 4139 El Camino Way, Palo Alto, California 94306, USA. p. 249-279.
6. Hall, J., K.L. Haas, and J.H. Freedman, Role of MTL-1, MTL-2, and CDR-1 in Mediating Cadmium Sensitivity in Caenorhabditis elegans. Toxicological Sciences, 2012. 128(2): p. 418-426.
7. Hughes, S.L., et al., The Metabolomic Responses of Caenorhabditis elegans to Cadmium Are Largely Independent of Metallothionein Status, but Dominated by Changes in Cystathionine and Phytochelatins. Journal of Proteome Research, 2009. 8(7): p. 3512-3519.
8. Freeman, J.L., et al., Constitutively elevated salicylic acid signals glutathione-mediated nickel tolerance in Thlaspi nickel hyperaccumulators. Plant Physiology, 2005. 137(3): p. 1082-1091.
9. Mishra, S., et al., Phytochelatin synthesis and response of antioxidants during cadmium stress in Bacopa monnieri L. Plant Physiology and Biochemistry, 2006. 44(1): p. 25-37.
10. Li, Y.J., et al., Overexpression of phytochelatin synthase in Arabidopsis leads to enhanced arsenic tolerance and cadmium hypersensitivity (vol 45, pg 1787, 2004). Plant and Cell Physiology, 2005. 46(2): p. 387-387.
11. Rauser, W.E., Structure and function of metal chelators produced by plants - The case for organic acids, amino acids, phytin, and metallothioneins. Cell Biochemistry and Biophysics, 1999. 31(1): p. 19-48.
12. Zenk, M.H., Heavy metal detoxification in higher plants - A review. Gene, 1996. 179(1): p. 21-30.
13. Pivato, M., M. Fabrega-Prats, and A. Masi, Low-molecular-weight thiols in plants: Functional and analytical implications. Archives of Biochemistry and Biophysics, 2014. 560: p. 83-99.
14. Meuwly, P., P. Thibault, and W.E. Rauser, GAMMA-GLUTAMYLCYSTEINYLGLUTAMIC ACID - A NEW HOMOLOG OF GLUTATHIONE IN MAIZE SEEDLINGS EXPOSED TO CADMIUM. Febs Letters, 1993. 336(3): p. 472-476.
15. Kubota, H., et al., Phytochelatin homologs induced in hairy roots of horseradish. Phytochemistry, 2000. 53(2): p. 239-245.
16. Ha, S.B., et al., Phytochelatin synthase genes from arabidopsis and the yeast Schizosaccharomyces pombe. Plant Cell, 1999. 11(6): p. 1153-1163.
17. Vatamaniuk, O.K., et al., AtPCS1, a phytochelatin synthase from Arabidopsis: Isolation and in vitro reconstitution. Proceedings of the National Academy of Sciences of the United States of America, 1999. 96(12): p. 7110-7115.
18. Clemens, S., et al., Tolerance to toxic metals by a gene family of phytochelatin synthases from plants and yeast. Embo Journal, 1999. 18(12): p. 3325-3333.
19. Matsumoto, S., et al., Functional analysis of phytochelatin synthase from Arabidopsis thaliana and its expression in Escherichia coli and Saccharomyces cerevisiae. Science and Technology of Advanced Materials, 2004. 5(3): p. 377-381.
20. Hayashi, Y., et al., 2 PATHWAYS IN THE BIOSYNTHESIS OF CADYSTINS (LAMBDA-EC)NG IN THE CELL-FREE SYSTEM OF THE FISSION YEAST. Biochemistry and Cell Biology-Biochimie Et Biologie Cellulaire, 1991. 69(2-3): p. 115-121.
21. Wunschmann, J., et al., Phytochelatins are synthesized by two vacuolar serine carboxypeptidases in Saccharomyces cerevisiae. Febs Letters, 2007. 581(8): p. 1681-1687.
22. Suresh, B. and G.A. Ravishankar, Phytoremediation - A novel and promising approach for environmental clean-up. Critical Reviews in Biotechnology, 2004. 24(2-3): p. 97-124.
23. Lee, J.H., An overview of phytoremediation as a potentially promising technology for environmental pollution control. Biotechnology and Bioprocess Engineering, 2013. 18(3): p. 431-439.
24. Jozefczak, M., et al., Differential response of Arabidopsis leaves and roots to cadmium: Glutathione-related chelating capacity vs antioxidant capacity. Plant Physiology and Biochemistry, 2014. 83: p. 1-9.
25. Wojcik, M. and A. Tukiendorf, Accumulation and tolerance of lead in two contrasting ecotypes of Dianthus carthusianorum. Phytochemistry, 2014. 100: p. 60-65.
26. Lopez-Climent, M.F., et al., Effect of cadmium and calcium treatments on phytochelatin and glutathione levels in citrus plants. Plant Biology, 2014. 16(1): p. 79-87.
27. Postrigan, B.N., et al., Effect of cadmium on promoter activity of rice phytochelatin synthase gene in transgenic tobacco plants. Russian Journal of Plant Physiology, 2013. 60(5): p. 701-705.
28. Gupta, D.K., H.G. Huang, and F.J. Corpas, Lead tolerance in plants: strategies for phytoremediation. Environmental Science and Pollution Research, 2013. 20(4): p. 2150-2161.
29. Cobbett, C. and P. Goldsbrough, Phytochelatins and metallothioneins: Roles in heavy metal detoxification and homeostasis. Annual Review of Plant Biology, 2002. 53: p. 159-182.
30. Cobbett, C.S., Phytochelatins and their roles in heavy metal detoxification. Plant Physiology, 2000. 123(3): p. 825-832.
31. Cobbett, C.S., Heavy metal detoxification in plants: Phytochelatin biosynthesis and function. Iubmb Life, 2001. 51(3): p. 183-188.
32. Zagorchev, L., et al., A Central Role for Thiols in Plant Tolerance to Abiotic Stress. International Journal of Molecular Sciences, 2013. 14(4): p. 7405-7432.
33. Li, Y.J., et al., Overexpression of phytochelatin synthase in Arabidopsis leads to enhanced arsenic tolerance and cadmium hypersensitivity. Plant and Cell Physiology, 2004. 45(12): p. 1787-1797.
34. Stolt, J.P., et al., Phytochelatin and cadmium accumulation in wheat. Environmental and Experimental Botany, 2003. 49(1): p. 21-28.
35. Rigouin, C., et al., Characterization of the phytochelatin synthase from the human parasitic nematode Ancylostoma ceylanicum. Molecular and Biochemical Parasitology, 2013. 191(1): p. 1-6.
36. Liebeke, M., et al., Earthworms Produce phytochelatins in Response to Arsenic. Plos One, 2013. 8(11).
37. Bundy, J.G., et al., Metallothioneins May Not Be Enough-The Role of Phytochelatins in Invertebrate Metal Detoxification. Environmental Science & Technology, 2014. 48(2): p. 885-886.
38. Cui, Y., et al., Toxicogenomic analysis of Caenorhabditis elegans reveals novel genes and pathways involved in the resistance to cadmium toxicity. Genome Biology, 2007. 8(6).
39. Schwartz, M.S., et al., Detoxification of Multiple Heavy Metals by a Half-Molecule ABC Transporter, HMT-1, and Coelomocytes of Caenorhabditis elegans. Plos One, 2010. 5(3).
40. Vatamaniuk, O.K., et al., CeHMT-1, a putative phytochelatin transporter, is required for cadmium tolerance in Caenorhabditis elegans. Journal of Biological Chemistry, 2005. 280(25): p. 23684-23690.
41. Wood, B.A. and J. Feldmann, Quantification of phytochelatins and their metal(loid) complexes: critical assessment of current analytical methodology. Analytical and Bioanalytical Chemistry, 2012. 402(10): p. 3299-3309.
42. Ju, X.H., et al., Determination and characterization of cysteine, glutathione and phytochelatins (PC2-6) in Lolium perenne L. exposed to Cd stress under ambient and elevated carbon dioxide using HPLC with fluorescence detection. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences, 2011. 879(20): p. 1717-1724.
43. Kawakami, S.K., M. Gledhill, and E.P. Achterberg, Determination of phytochelatins and glutathione in phytoplankton from natural waters using HPLC with fluorescence detection. Trac-Trends in Analytical Chemistry, 2006. 25(2): p. 133-142.
44. Vacchina, V., et al., Characterisation and determination of phytochelatins in plant extracts by electrospray tandem mass spectrometry. Analyst, 1999. 124(10): p. 1425-1430.
45. Baralkiewicz, D., et al., Determination of cadmium and lead species and phytochelatins in pea (Pisum sativum) by HPLC-ICP-MS and HPLC-ESI-MSn. Talanta, 2009. 79(2): p. 493-498.
46. Brautigam, A., et al., Rapid and simple UPLC-MS/MS method for precise phytochelatin quantification in alga extracts. Analytical and Bioanalytical Chemistry, 2010. 398(2): p. 877-883.
47. Zitka, O., et al., Rapid and Ultrasensitive Method for Determination of Phytochelatin(2) using High Performance Liquid Chromatography with Electrochemical Detection. International Journal of Electrochemical Science, 2011. 6(5): p. 1367-1381.
48. Zitka, O., et al., Phytochelatin synthase activity as a marker of metal pollution. Journal of Hazardous Materials, 2011. 192(2): p. 794-800.
49. Nakazawa, R., et al., Optimum assay conditions of the activity of phytochelatin synthase from tobacco cells. Biologia Plantarum, 2002. 45(2): p. 311-313.
50. Ogawa, S., et al., HPLC method for the determination of phytochelatin synthase activity specific for soft metal ion chelators. Journal of Inorganic Biochemistry, 2010. 104(4): p. 442-445.
J.Met.Nano:
volume-1, issue-3
- Human papilloma virus (HPV) and methods for its identification in head and neck cancers
- Clinical application of capillary electrophoresis – determination of free amino acids in body fluids
- The role of phytochelatins in plant and animals: A review
- Paramagnetic particles for immobilization of metallothionein – promising biomarker of head and neck cancer
- Delivery of doxorubicin using protein nanocarriers
- MIR-150 electrochemical detection connected with specific isolation based on magnetic particles
- Nanomaghemite core functionalized with ion-exchange resins for isolation of biogennic amines
- Modern techniques of increase the antibacterial properties of the instruments
- Detection of sentinel lymph node using magnetic nanoparticles
- Opening of working and meeting room for nanobiometalnet project
- New directions of electrochemistry, bioelectrochemistry,nanoelectrochemistry and bioengineering
 PDF
PDF