
Clinical application of capillary electrophoresis – determination of free amino acids in body fluids
Markéta Janotová, Kristýna Šmerková, Markéta Vaculovičová, René Kizek
Determination of biomarkers in body fluids provides information about the overall state of the organism 1. Among commonly determined markers in clinical laboratories belong AAs. The AAs (Fig. 1), basic structural units of peptides, as well as their derivatives occupy many metabolic and biochemical roles in human body 2,3. The AAs belong to zwitterions containing acidic and basic functional groups (carboxyl groups and amino, respectively). Zwitterions have neutral character at a certain pH known as the isoelectric point due the dissociation of acidic and basic groups. Therefore, the charge of AAs depend on the number of functional groups and pH environment 4.
Determination of AAs levels in the body fluids such as blood and/or plasma 5,6, cerebrospinal fluid 6,7, urine 6,8, saliva 6,7 or amniotic fluid 9 represents the significant clinical indicator not only for control of the nutritional state of the organism, but for a number of metabolic disorders 10. Examples can be inherited metabolic disorders such as phenylketonuria (PKU) (an enzyme deficiency in phenylalanine hydroxylase (PAH) (Fig. 2)) 11 or maple syrup urine disease (an enzyme deficiency in branched-chain α-ketoacid dehydrogenase (BCKD) (Fig. 3)) 12.
There are many methods for AAs determination employing various techniques such as high pressure liquid chromatography (HPLC) 13 or gas chromatography (GC) 14. During the 80's of the 20th century CE became a routine laboratory technique 15. Recently, this method has ranked among very useful tools for separation and determination of AAs 2,9,16,17, biogenic amines 18 or peptides 19 and is often used as an alternative to HPLC or GC due to its electrophoretic separation mechanism 16,20. In addition, a main advantage is very low sample and solvent consumption, speed of analysis and excellent separation efficiency 2,16,21-23. Besides, due to the high resolving power is CE a promising method for the analysis of biological fluids (complex mixtures of many metabolites) 2,22.
The essential part of the CE instrumentation is a detector. In photometric and fluorescence detection is problem with a limited presence of chromophores and fluorofores. Because of that, the derivatization procedures prior CE are usually used, which is the one of the limiting factors 24. Therefore, there are attempts to analyze AAs without derivatization employing various strategies such as indirect detection 25, electrochemical detection (conductometric detection 6,8, amperometric detection 26) or CE in combination with mass spectrometry (MS) 5,11.
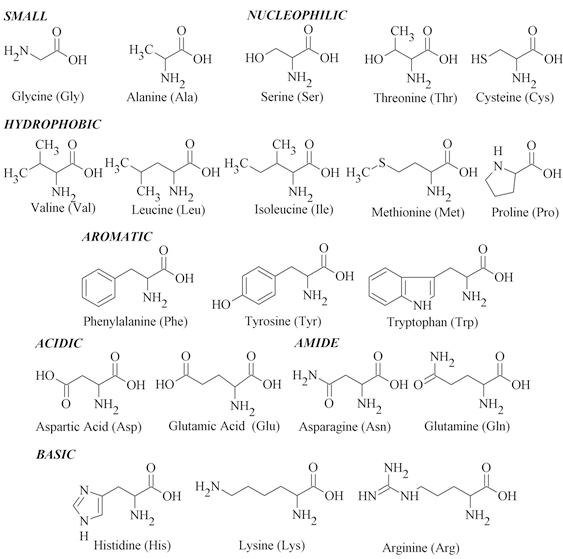
Figure 1: Twenty commonly occurring amino acids
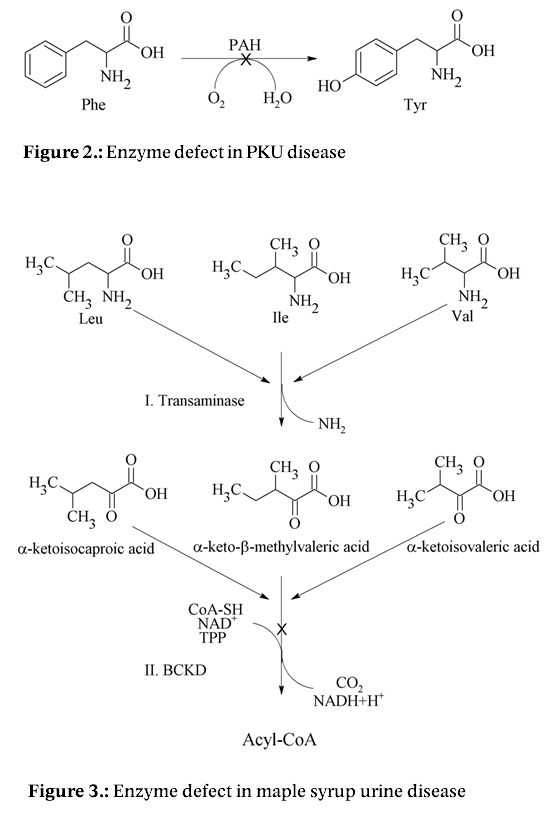
Figure 2: Enzyme defect in maple syrup urine disease
1. Senk P., Kozak L., Foret F.: Electrophoresis, 25, 1447 (2004).
2. Strieglerova L., Kuban P., Bocek P.: J. Chromatogr. A, 1218, 6248 (2011).
3. Zunic G., Jelic-Ivanovic Z., Colic M., Spasic S.: J. Chromatogr. B, 772, 19 (2002).
4. Narezhnaya E. V., Askalepova O. I., Nikashina A. A., Krukier, II, Pogorelova T. N.: J. Anal. Chem., 65, 1280 (2010).
5. Martin-Girardeau A., Renou-Gonnord M. F.: J. Chromatogr. B, 742, 163 (2000).
6. Tuma P., Stulik K.: Methods in molecular biology (Clifton, N.J.), 919, 13 (2013).
7. Deng Y. H., Wang H., Zhang H. S.: J. Sep. Sci., 31, 3088 (2008).
8. Madr A., Cela A., Musilova J., Zeisbergerova M., Glatz Z.: Chem. Listy, 107, S318 (2013).
9. Tuma P., Samcova E., Andelova K.: J. Chromatogr. B, 839, 12 (2006).
10. Lorenzo M. P., Villasenor A., Ramamoorthy A., Garcia A.: Electrophoresis, 34, 1701 (2013).
11. Jeong J. S., Kim S. K., Park S. R.: Anal. Bioanal. Chem., 405, 8063 (2013).
12. Jeong J. S., Sim H. J., Lee Y. M., Yoon H. R., Kwon H. J., Hong S. P.: J. Chromatogr. B, 879, 2171 (2011).
13. Xiao Y., Tan T. T. Y., Ng S. C.: Analyst, 136, 1433 (2011).
14. Husek P., Macek K.: J Chromatogr, 113, 139 (1975).
15. Jorgenson J. W., Lukacs K. D.: J Chromatogr, 218, 209 (1981).
16. Pobozy E., Czarkowska W., Trojanowicz M.: J. Biochem. Biophys. Methods, 67, 37 (2006).
17. Van Hemelrijek A., Sarre S., Smolders I., Michotte Y.: J. Neurosci. Methods, 144, 63 (2005).
18. Swann L. M., Forbes S. L., Lewis S. W.: Talanta, 81, 1697 (2010).
19. Moini M.: Methods in molecular biology (Clifton, N.J.), 276, 253 (2004).
20. Li Z., Zhang Y., Tong F. H., Jiang T. T., Zheng H. P., Ye J. N., Chu Q. C.: Chin. Chem. Lett., 25, 640 (2014).
21. Coufal P., Zuska J., van de Goor T., Smith V., Gas B.: Electrophoresis, 24, 671 (2003).
22. Kaneta T., Maeda H., Miyazaki M., Miyake R., Izaki H., Sakoda Y., Kinoshita S., Imasaka T.: J. Chromatogr. Sci., 46, 712 (2008).
23. Samcova E., Tuma P.: Electroanalysis, 18, 152 (2006).
24. Zunic G., Spasic S.: J. Chromatogr. B, 873, 70 (2008).
25. Zunic G. D., Spasic S., Jelic-Ivanovic Z.: Amino Acid Analysis: Methods and Protocols, 828, 243 (2012).
26. Guo Y., Colon L. A., Dadoo R., Zare R. N.: Electrophoresis, 16, 493 (1995).
27. Hoekstra R., Fekkes D., Loonen A. J. M., Pepplinkhuizen L., Tuinier S., Verhoeven W. M. A.: Eur. Neuropsychopharmacol., 16, 71 (2006).
28. Schaffer A., Verdoia M., Cassetti E., Marino P., Suryapranata H., De Luca G., Novara Atherosclerosis Study G.: Thromb. Res., 134, 288 (2014).
29. El-Khairy L., Ueland P. M., Refsum H., Graham I. M., Vollset S. E.: Circulation, 103, 2544 (2001).
30. Zinellu A., Sotgia S., Posadino A. M., Pasciu V., Perino M. G., Tadolini B., Deiana L., Carru C.: Electrophoresis, 26, 1063 (2005).
31. Mitani H., Shirayama Y., Yamada T., Maeda K., Ashby C. R., Kawahara R.: Prog. Neuro-Psychopharmacol. Biol. Psychiatry, 30, 1155 (2006).
32. Maes M., Debacker G., Suy E., Minner B.: Neuropsychobiology, 31, 10 (1995).
33. Clague A., Thomas A.: Clin. Chim. Acta, 315, 99 (2002).
34. Hanley W. B., Demshar H., Preston M. A., Borczyk A., Schoonheyt W. E., Clarke J. T. R., Feigenbaum A.: Early Hum. Dev., 47, 87 (1997).
35. Schulze A., Mayatepek E., Hoffmann G. F.: Clin. Chim. Acta, 317, 27 (2002).
36. Allard P., Cowell L. D., Zytkovicz T. H., Korson M. S., Ampola M. G.: Clin. Biochem., 37, 857 (2004).
37. Ruzicka B. B., Jhamandas K. H.: Prog. Neurobiol., 40, 223 (1993).
38. Lu M. J., Chiu T. C., Chang P. L., Ho H. T., Chang H. T.: Anal. Chim. Acta, 538, 143 (2005).
39. Sandlin Z. D., Shou M. S., Shackman J. G., Kennedy R. T.: Anal. Chem., 77, 7702 (2005).
40. Krogsgaardlarsen P., Wahl P., Schousboe A., Madsen U., Hansen J. J.: Biochem. Soc. Trans., 21, 102 (1993).
41. Piepponen T. P., Skujins A.: J. Chromatogr. B, 757, 277 (2001).
42. Shah A. J., Crespi F., Heidbreder C.: J. Chromatogr. B, 781, 151 (2002).
43. JimenezJimenez F. J., Molina J. A., Vargas C., Gomez P., Navarro J. A., BenitoLeon J., OrtiPareja M., Gasalla T., Cisneros E., Arenas J.: J. Neurol. Sci., 141, 39 (1996).
44. Skvortsova V. I., Raevsky K. S., Kovalenko A. V., Kudrin V. S., Malikova L. A., Sokolov M. A., Alekseev A. A., Gusev E. I.: Zhurnal Nevropatol. Psikhiatrii Im. S S Korsakova, 99, 34 (1999).
45. Brouns R., Van Hemelrijck A., Drinkenburg W. H., Van Dam D., De Surgeloose D., De Deyn P. P.: Neurochem. Int., 56, 865 (2010).
46. Assaf B. A., Rothman D. L., Novotny E. J.: Neurology, 46, 6058 (1996).
47. Kesel R. G., Odonnell J. F., Kirch E. R., Wach E. C.: American Journal of Orthodontics and Oral Surgery-Oral Surgery, 33, 68 (1947).
48. Battistone G. C., Burnett G. W.: Arch. Oral Biol., 3, 161 (1961).
49. Nakamura Y., Kodama H., Satoh T., Adachi K., Watanabe S., Yokote Y., Sakagami H.: In Vivo, 24, 837 (2010).
50. Rausch-Fan X. H., Ulm C., Jensen-Jarolim E., Schedle A., Boltz-Nitulescu G., Rausch W. D., Matejka M.: J. Periodont., 76, 1182 (2005).
51. Linkosalo E., Markkanen H., Syrjanen S.: J. Nutr., 115, 588 (1985).
52. Mayboroda O. A., Neususs C., Pelzing M., Zurek G., Derks R., Meulenbelt I., Kloppenburg M., Slagboom E. P., Deelder A. M.: J. Chromatogr. A, 1159, 149 (2007).
53. Finegan J. A. K.: Br. J. Obstet. Gynaecol., 91, 745 (1984).
54. Stewart C. J., Iles R. K., Perrett D.: Electrophoresis, 22, 1136 (2001).
55. Chen H., Xu Y., Ip M. P. C.: J. Liq. Chromatogr. Relat. Technol., 20, 2475 (1997).
56. Tak Y. H., Somsen G. W., de Jong G. J.: Anal. Bioanal. Chem., 401, 3275 (2011).
57. Ramautar R., Mayboroda O. A., Derks R. J. E., van Nieuwkoop C., van Dissel J. T., Sornsen G. W., Deelder A. M., de Jong G. J.: Electrophoresis, 29, 2714 (2008).
J.Met.Nano:
volume-1, issue-3
- Human papilloma virus (HPV) and methods for its identification in head and neck cancers
- Clinical application of capillary electrophoresis – determination of free amino acids in body fluids
- The role of phytochelatins in plant and animals: A review
- Paramagnetic particles for immobilization of metallothionein – promising biomarker of head and neck cancer
- Delivery of doxorubicin using protein nanocarriers
- MIR-150 electrochemical detection connected with specific isolation based on magnetic particles
- Nanomaghemite core functionalized with ion-exchange resins for isolation of biogennic amines
- Modern techniques of increase the antibacterial properties of the instruments
- Detection of sentinel lymph node using magnetic nanoparticles
- Opening of working and meeting room for nanobiometalnet project
- New directions of electrochemistry, bioelectrochemistry,nanoelectrochemistry and bioengineering
 PDF
PDF