
Paramagnetic particles for immobilization of metallothionein – promising biomarker of head and neck cancer
Natalia Cernei, Zbyněk Heger, Kateřina Tmejová, Roman Guráň, Pavel Kopel, Ondřej Zítka, Vojtěch Adam, René Kizek
Metallothioneins (MTs) are a group of low-molecular weight (~6 -10kDa), cysteine-rich, evolutionarily conserved proteins, found in generally all life forms. The MTs are multi-functional and play crucial roles in the transport of essential trace elements as well as in the detoxification of harmful metallic elements 1. Four major MT isoforms, MT-1, MT-2, MT-3 and MT-4, have been identified in mammals 2. Specifically, MT-3 has been reported to be secreted mainly in neural cells. Recent reports established that this isoform play an important protective role in brain injury and metal-linked neurodegenerative diseases 3. In general, MT is known to modulate three basic processes: the release of hydroxyl radical or nitric oxide 4, apoptosis 5 and the binding and exchange of heavy metals such as zinc 6, cadmium 7 or copper 8. Previous studies have shown a positive correlation between the expression of MT with invasion, metastasis and poor prognosis in various cancers, including head and neck cancers 9, where increasing levels of MT concentration in tumor (vs. healthy tissues) was observed 10. Moreover; due to high affinity towards heavy metals, MT can be employed for modification of electrodes in development of electrochemical biosensors 11. In view of these facts, immobilization of MT on paramagnetic particles (PMPs) may provide many possibilites, such as simplification of biosensing of low levels of MT, through its pre-concentration. The present study demonstrates immobilization of MT on a surface of paramagnetic particles (gold modified). For confirmation of MT presence two detection techniques were employed: matrix-assisted laser desorption/ionization - time of flight mass spectrometry and differential pulse voltammetry with adsorptive transfer technique utilizing Brdicka solution as supporting electrolyte. For binding capacity evaluation MT isolated from rabbit liver was employed and ideal particles for its immobilization were chosen, simultaneously with optimization of immobilization conditions (pH and temperature).
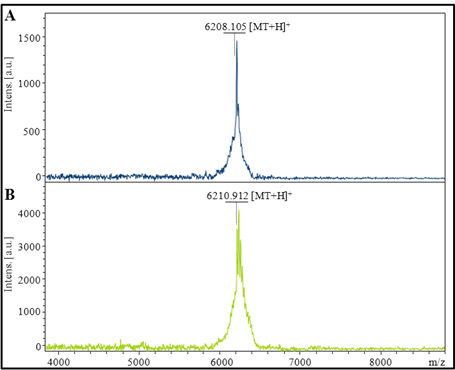
Figure 1. MALDI-TOF/TOF mass spectra of MT fractions after the fast protein liquid chromatography purification (concentration of MT - 2.0 μM). In analyses the linear positive mode, (A) HCCA and (B) DHB matrixes were employed.
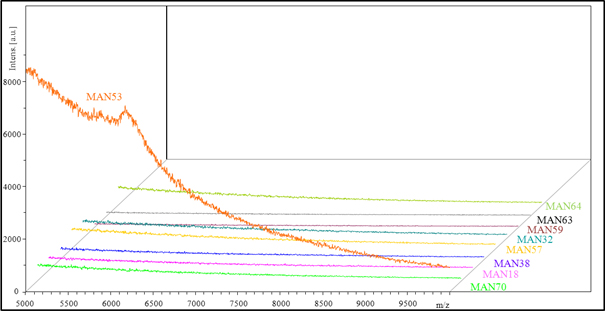
Figure 2. MALDI-TOF/TOF mass spectra showing the results of screening of various types of paramagnetic particles (MANX) for their ability to bind MT (1 µM) on their surface. MS measurements were carried out in linear positive ion mode, with DHB matrix and laser power established to 80%.
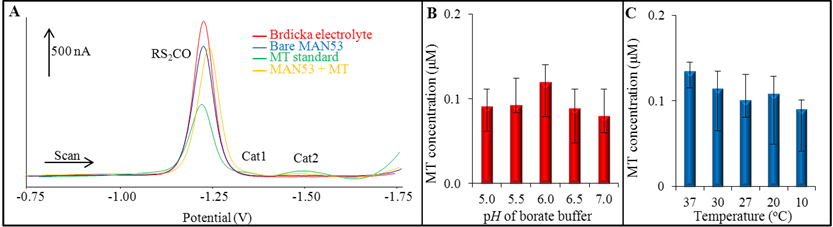
Figure 3. (A) Typical voltammogram, obtained by using AdT DPV with Brdicka electrolyte, where (a) stays for Brdicka electrolyte (1 mM Co(NH3)6Cl3 and 1 M ammonia buffer (NH3(aq) and NH4Cl, pH = 9.6), (b) for bare MAN53, (c) for MT standard (500 nM) and (d) for MAN53 + MT conjugate. (B) Optimization of ideal binding conditions – pH of binding borate buffer (5.0 - 7.0). (C) Influence of temperature (10 – 37oC) on binding of MT on MAN53. All optimization conditions were evaluated by using AdT DPV. Values are means of three independent replicates (n = 3). Vertical bars indicate standard error.
1. Wang, Y.H.; Zhao, Z.; Zhang, L.P.; Hu, C.Q.; Ren, C.H.; Yuan, L.H., Molecular characterization of metallothionein from white shrimp, Litopenaeus vannamei and its expression response to salinity stress. Marine Biology Research 2014, 10, 731-737.
2. Theocharis, S.E.; Margeli, A.P.; Koutselinis, A., Metallothionein: a multifunctional protein from toxicity to cancer. Int J Biol Markers 2003, 18, 162-169.
3. Vasak, M.; Meloni, G., Chemistry and biology of mammalian metallothioneins. J Biol Inorg Chem 2011, 16, 1067-1078.
4. Andrews, G.K., Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochemical Pharmacology 2000, 59, 95-104.
5. Dutsch-Wicherek, M.; Sikora, J.; Tomaszewska, R., The possible biological role of metallothionein in apoptosis. Front Biosci 2008, 13, 4029-4038.
6. Torreggiani, A.; Domenech, J.; Atrian, S.; Capdevila, M.; Tinti, A., Raman study of in vivo synthesized Zn(II)-metallothionein complexes: structural insight into metal clusters and protein folding. Biopolymers 2008, 89, 1114-1124.
7. Orihuela, R.; Domenech, J.; Bofill, R.; You, C.; Mackay, E.A.; Kagi, J.H.; Capdevila, M.; Atrian, S., The metal-binding features of the recombinant mussel Mytilus edulis MT-10-IV metallothionein. J Biol Inorg Chem 2008, 13, 801-812.
8. Bulcke, F.; Dringen, R., Copper Oxide Nanoparticles Stimulate Glycolytic Flux and Increase the Cellular Contents of Glutathione and Metallothioneins in Cultured Astrocytes. Neurochem Res 2014, 26, 26.
9. Cherian, M.G.; Howell, S.B.; Imura, N.; Klaassen, C.D.; Koropatnick, J.; Lazo, J.S.; Waalkes, M.P., Role of metallothionein in carcinogenesis. Toxicol Appl Pharmacol 1994, 126, 1-5.
10. Gumulec, J.; Raudenska, M.; Adam, V.; Kizek, R.; Masarik, M., Metallothionein - immunohistochemical cancer biomarker: a meta-analysis. PLoS One 2014, 9.
11. Trnkova, L.; Krizkova, S.; Adam, V.; Hubalek, J.; Kizek, R., Immobilization of metallothionein to carbon paste electrode surface via anti-MT antibodies and its use for biosensing of silver. Biosensors & Bioelectronics 2011, 26, 2201-2207.
12. Skalickova, S.; Zitka, O.; Nejdl, L.; Krizkova, S.; Sochor, J.; Janu, L.; Ryvolova, M.; Hynek, D.; Zidkova, J.; Zidek, V., et al., Study of Interaction between Metallothionein and CdTe Quantum Dots. Chromatographia 2013, 76, 345-353.
13. Tmejova, K.; Hynek, D.; Kopel, P.; Krizkova, S.; Blazkova, I.; Trnkova, L.; Adam, V.; Kizek, R., Study of metallothionein-quantum dots interactions. Colloids and Surfaces B-Biointerfaces 2014, 117, 534-537.
14. Heger, Z.; Cernei, N.; Guran, R.; Michalek, P.; Milosavljevic, V.; Kopel, P.; Zitka, O.; Kynicky, J.; Lany, P.; Adam, V., et al., gamma-Fe2O3 Magnetic Core Functionalized with Tetraethyl Orthosilicate and 3-Aminopropyl Triethoxysilane for an Isolation of H7N7 Influenza Serotype Virions. International Journal of Electrochemical Science 2014, 9, 3374-3385.
15. Zitka, O.; Cernei, N.; Heger, Z.; Matousek, M.; Kopel, P.; Kynicky, J.; Masarik, M.; Kizek, R.; Adam, V., Microfluidic chip coupled with modified paramagnetic particles for sarcosine isolation in urine. Electrophoresis 2013, 34, 2639-2647.
16. Zhou, M.; Wang, B.X.; Rozynek, Z.; Xie, Z.H.; Fossum, J.O.; Yu, X.F.; Raaen, S., Minute synthesis of extremely stable gold nanoparticles. Nanotechnology 2009, 20.
17. Metallothioneins and Related Chelators. In Metallothioneins and Related Chelators, Sigel, A.; Sigel, H.; Sigel, R.K.O., Eds. Royal Soc Chemistry, Thomas Graham House, Science Park, Cambridge Cb4 4wf, Cambs, Uk: 2009; Vol. 5.
18. Saito, S.; Kurasaki, M., Gold replacement of cadmium, zinc-binding metallothionein. Research Communications in Molecular Pathology and Pharmacology 1996, 93, 101-107.
19. Erk, M.; Raspor, B., Anodic stripping voltammetry in the complexation study of the peptide Lys-Cys-Thr-Cys-Cys-Ala 56-61 MT I and cadmium: application in determination of the complexing capacity and stability constant. Journal of Electroanalytical Chemistry 2001, 502, 174-179.
20. Raspor, B.; Paic, M.; Erk, M., Analysis of metallothioneins by the modified Brdicka procedure. Talanta 2001, 55, 109-115.
J.Met.Nano:
volume-1, issue-4
- Human papilloma virus (HPV) and methods for its identification in head and neck cancers
- Clinical application of capillary electrophoresis – determination of free amino acids in body fluids
- The role of phytochelatins in plant and animals: A review
- Paramagnetic particles for immobilization of metallothionein – promising biomarker of head and neck cancer
- Delivery of doxorubicin using protein nanocarriers
- MIR-150 electrochemical detection connected with specific isolation based on magnetic particles
- Nanomaghemite core functionalized with ion-exchange resins for isolation of biogennic amines
- Modern techniques of increase the antibacterial properties of the instruments
- Detection of sentinel lymph node using magnetic nanoparticles
- Opening of working and meeting room for nanobiometalnet project
- New directions of electrochemistry, bioelectrochemistry,nanoelectrochemistry and bioengineering
 PDF
PDF