
Modern techniques of increase the antibacterial properties of the instrument
Kristýna Číhalová, Dagmar Chudobová, Vedran Milosavljevic, Pavel Kopel, Zbyněk Heger, Vojtěch Adam, René Kizek
Nowadays food and textile industries as well as health centers have employed advanced technologies and precaution measures which prevent bacteria proliferation and distribution among staff, consumers and patients. 1-3. These technologies and measures aren’t always effective to fulfill the aseptic requirements. One of the main objectives to aseptic conditions is related to the necessity for the treatment of surfaces, tools and working materials by decontaminants. The application of chemotherapeutics or antibiotics isn’t always a good choicesince they may be in direct contact with the product, or may migrate and cause the transfer of contaminants into the product 3. Some organic compounds used for desinfection purposes pose some disadvantages, including their toxicity for the human body 4. Thereofore there is an increasing of interest in using of inoarganic disenfectants, such as metal nanoparticles (NPs)5. Furthermore, a crucial factor related to the use of chemotherapeutics and antibiotics is the enhancement of resistant bacteria toward these decontaminant agents 6. Therefore it’s necessary to develop new compounds wich would show baktericide properties also toward bacterial species immuned with special resistance or multiresistance to antibiotics 6, 7. Bacteria with enhanced resistance are often found in hospital operations as a consequence of long-term antibiotics administration to patients, leading to genetic mutations and formation of various defense systems against damaging mechanism of antibiotics 8. The most resistant form which evolved from the bacteria S. aureus is methicilin-resistant S. aureus (MRSA). Enhanced resistance to antibiotics or other bactericidal agents in staphylococ is present due to the variable genetic element such as pathogenicity islands genome, plasmids, transpons and others. The responsible gene for resistance enhancement in MRSA if found in the staphyloccocal chromosomal cassette mec (SCCmec) 9.
The integration of SCCmec into the genome of S. aureus leads to methicillin-resistant strain (MRSA) which is resistant to nearly all β-lactam antibiotics. The resistance toward methicillin is dedicated to the presence of penicillin-binding protein 2a (PBP2a) and the β-lactamase enzyme that cleaves β-lactam antibiotics (penicillin-based antibiotics), and is encoded by the mecA gene 10. The PBP2a is an analogue of PBP transpeptidases whose presence is essential for normal cell, as they play an important role in cell-wall synthesis 11. The presence of β-lactam antibiotics inhibit their function, and the cell loses its ability to produce cell wall and consequently dies. However PBP2a exhibits a low affinity toward β-lactam antibiotics, therefore is not subject to their inhibition 9. All of this lead to cost uneffective and time consuming therapy for hospitalized patients with MRSA 12.The treatment of surfaces, tools, fabrics or packages with antibacterial agents in the above mentioned areas is an absolute necessity, and it is therefore it is necessary to ensure an effective treating agent with pronounced antibacterial effect. Such a property is prone of metal nanoparticles 13, graphene oxide (GO) or biofunctionalized graphene 14. Graphene with antimicrobial effect is used in containers for food packaging or for the coating of biomedical devices, wherein the bacterial colonization on the surface are unwanted 15. Modification of graphene metal nanoparticles with antibacterial effect streamlines this effect.
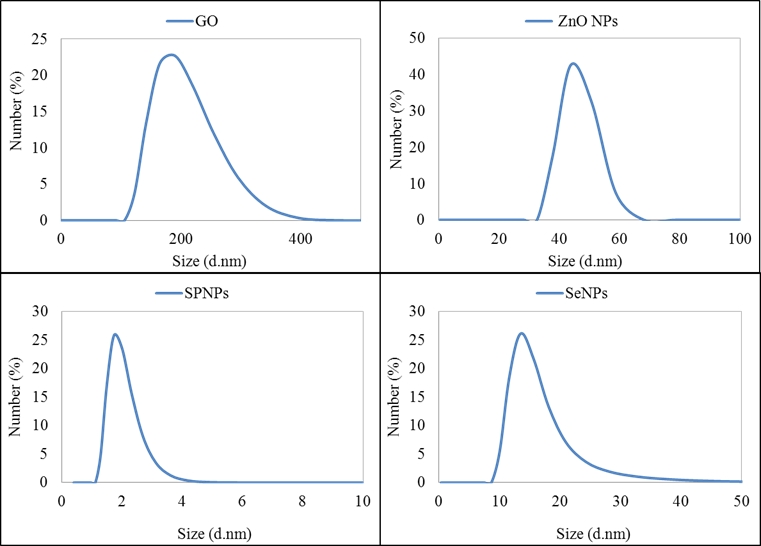
Fig. 1: Results of measurment of particle size by using dynamic light scattering.
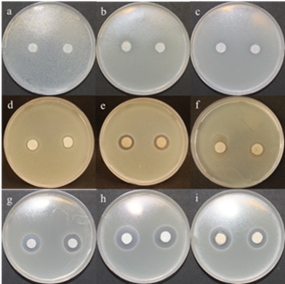
Fig. 2: Testing the antibacterial action by method of inhibition zones. While we tested GO modified by ZnO avoid to the formation of inhibition zones around any of the test bacterial cultures E. coli (a), S. aureus (b) a MRSA (c). In GO modified by SPNPs have been created 2 mm inhibition zone in E. coli (d), 3 mm in S. aureus (e) a 1 mm in MRSA (f). In modification GO by SeNPs particles leads to the formation of 4 mm inhibition in E. coli (g), 6 mm in S. aureus (h) and 5 mm in MRSA (i).
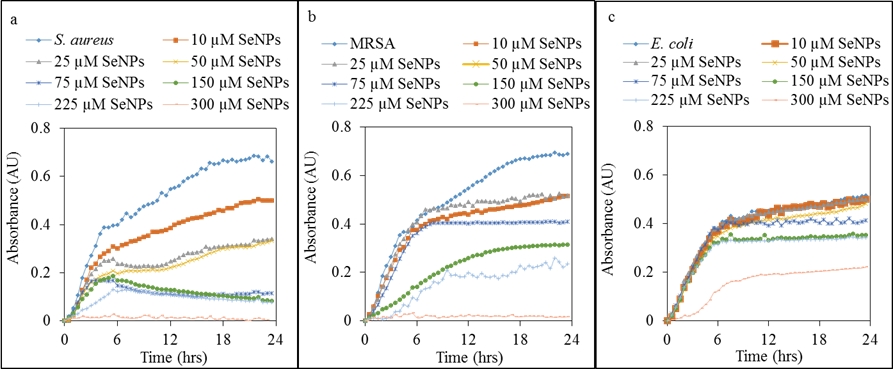
Fig. 3: Growth curves of bacterial cultures after application grephene oxide with different concentrations of selenium nanoparticles. Using concentration of grephene oxide was stacionary for all different concentrations of selenium nanoparticles. S. aureus (a) and MRSA (b) growth in a similar downward trend with increasing concentrations of selenium nanoparticles in complex with graphene oxide (1 mg/ml). For E. coli was not too significant downward trend (c).
1. Yip, J., et al., Investigation of Antifungal and Antibacterial Effects of Fabric Padded with Highly Stable Selenium Nanoparticles. Journal of Applied Polymer Science, 2014. 131(17).
2. Nateghi, M.R. and H. Hajimirzababa, Effect of silver nanoparticles morphologies on antimicrobial properties of cotton fabrics. Journal of the Textile Institute, 2014. 105(8): p. 806-813.
3. Olar, R., et al., Synthesis, characterisation and thermal behaviour of some thiosulfato- and sulfato copper(II) complexes - Antibacterial activity. Journal of Thermal Analysis and Calorimetry, 2008. 92(1): p. 245-251.
4. Messad, S., et al., Frequency of contamination and antimicrobial resistance of thermotolerant Campylobacter isolated from some broiler farms and slaughterhouses in the region of Algiers. Food Control, 2014. 40: p. 324-328.
5. Hajipour, M.J., et al., Antibacterial properties of nanoparticles. Trends in Biotechnology, 2012. 30(10): p. 499-511.
6. Chudobova, D., et al., Comparison of the effects of silver phosphate and selenium nanoparticles on Staphylococcus aureus growth reveals potential for selenium particles to prevent infection. Fems Microbiology Letters, 2014. 351(2): p. 195-201.
7. Panacek, A., et al., Silver colloid nanoparticles: Synthesis, characterization, and their antibacterial activity. Journal of Physical Chemistry B, 2006. 110(33): p. 16248-16253.
8. Thomas, R., et al., Antibacterial Activity and Synergistic Effect of Biosynthesized AgNPs with Antibiotics Against Multidrug-Resistant Biofilm-Forming Coagulase-Negative Staphylococci Isolated from Clinical Samples. Applied Biochemistry and Biotechnology, 2014. 173(2): p. 449-460.
9. Bhargava, K. and Y.F. Zhang, Characterization of methicillin-resistant coagulase-negative staphylococci (MRCoNS) in retail meat. Food Microbiology, 2014. 42: p. 56-60.
10. Khan, S., et al., Rapid optical determination of beta-lactamase and antibiotic activity. Bmc Microbiology, 2014. 14.
11. Wan, M.T. and C.C. Chou, Spreading of beta-lactam resistance gene (mecA) and methicillin-resistant Staphylococcus aureus through municipal and swine slaughterhouse wastewaters. Water research, 2014. 64: p. 288-95.
12. Hernandez-Porto, M., et al., Risk factors for development of methicillin-resistant Staphylococcus aureus-positive clinical culture in nasal carriers after decolonization treatment. American Journal of Infection Control, 2014. 42(7): p. E75-E79.
13. Kanmani, P. and S.T. Lim, Synthesis and characterization of pullulan-mediated silver nanoparticles and its antimicrobial activities. Carbohydrate Polymers, 2013. 97(2): p. 421-428.
14. Sang, J.K., et al., Composition useful for exhibiting antibacterial effect with respect to Gram-positive bacterium e.g. Streptococcus iniae or Gram-negative bacterium e.g. Escherichia coli, comprises graphene oxide, UNIV CHEJU NAT IND ACADEMIC COOP FOUND (UYCH-Non-standard).
15. Yadav, S.K., et al., Mechanically Robust, Electrically Conductive Biocomposite Films Using Antimicrobial Chitosan-Functionalized Graphenes. Particle & Particle Systems Characterization, 2013. 30(8): p. 721-727.
16. Leung, Y.H., et al., Antibacterial activity of ZnO nanoparticles with a modified surface under ambient illumination. Nanotechnology, 2012. 23(47).
17. Chudobova, D., et al., Complexes of Silver(I) Ions and Silver Phosphate Nanoparticles with Hyaluronic Acid and/or Chitosan as Promising Antimicrobial Agents for Vascular Grafts. International Journal of Molecular Sciences, 2013. 14(7): p. 13592-13614.
18. Zanzen, U., et al., Antibacterial action of copper ions on food-contaminating bacteria. Acta Biologica Szegediensis, 2013. 57(2): p. 149-151.
19. Kolar, S.L., et al., NsaRS is a Cell-Envelope-Stress Sensing Two-Component System of Staphylococcus aureus. ArrayExpress Archive, 2012.
20. Lee, S.J., et al., Electrospun chitosan nanofibers with controlled levels of silver nanoparticles. Preparation, characterization and antibacterial activity. Carbohydrate Polymers, 2014. 111: p. 530-537.
J.Met.Nano:
volume-1, issue-4
- Human papilloma virus (HPV) and methods for its identification in head and neck cancers
- Clinical application of capillary electrophoresis – determination of free amino acids in body fluids
- The role of phytochelatins in plant and animals: A review
- Paramagnetic particles for immobilization of metallothionein – promising biomarker of head and neck cancer
- Delivery of doxorubicin using protein nanocarriers
- MIR-150 electrochemical detection connected with specific isolation based on magnetic particles
- Nanomaghemite core functionalized with ion-exchange resins for isolation of biogennic amines
- Modern techniques of increase the antibacterial properties of the instruments
- Detection of sentinel lymph node using magnetic nanoparticles
- Opening of working and meeting room for nanobiometalnet project
- New directions of electrochemistry, bioelectrochemistry,nanoelectrochemistry and bioengineering
 PDF
PDF