
Human papilloma virus (HPV) and methods for its identification in head and neck cancers
Iva Blažková, Marie Konečná, Renáta Kenšová, Vojtěch Adam, René Kizek,
Human papillomavirus (HPV) selectively infects the epithelium of the skin and mucous membranes. Specific HPV types are associated with squamous cell carcinoma, adenocarcinoma, and dysplasias of the cervix, penis, anus, vagina and vulva1. The term head and neck cancer includes malignancy in an area that comprises the skin, oral cavity, salivary glands, lip, pharynx, larynx, nasal cavity, paranasal sinuses and soft tissues of the neck and ear2.
The first association of HPV with head and neck cancer was published in 1985 3. HPV was also shown to play a role in the pathogenesis of a subset of head and neck squamous cell carcinomas (HNSCCs)4. Almost 650,000 patients worldwide are diagnosed with head or neck cancer each year and 350,000 patients die of this disease as this cancer is the sixth most prevalent type of cancer worldwide. The ratio of males to females is approximately 2:12, 3. From the point of view of the infection, HPVs have developed several molecular mechanisms to enable infected cells to suppress apoptosis5, 6. Based on their potential for oncogenesis, HPV types can be classified both as high-risk or low-risk7. Precancerous lesions of the oral mucosa are epithelial changes that are able to undergo malignant transformation more likely than normal tissue at other mucosal sites8.
A total of 150 HPV genotypes have been identified9. The HPV 16 and 18 strains, which are known to cause nearly all cases of cervical cancer, also raise the risk of developing oropharyngeal cancer10. The evident similarities between both cervical and head and neck tumors prompted the utilization of the same HPV diagnostic procedures. There is now compelling evidence that specially designed methodologies must be employed for prognosis11, 12.
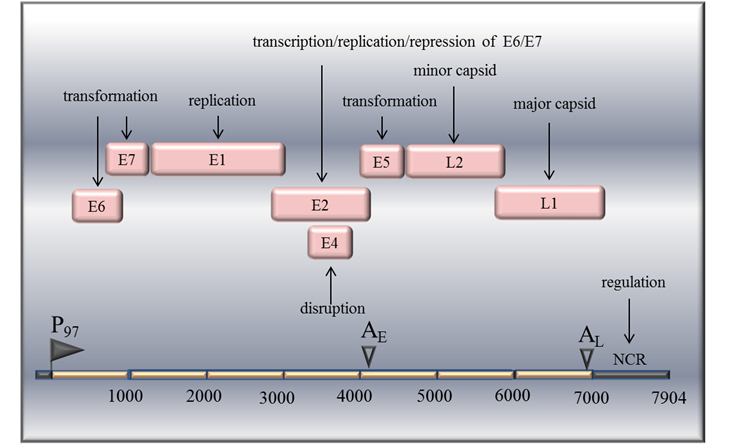
Figure 1. Genetic Map of the human papillomavirus (HPV) type 16. HPV genome is a double- -stranded circular DNA molecule. Early genes from the DNA sequence are designated E1 to E7 and late genes, L1, and L2, indicated in pink boxes. The non-coding region (NCR) is indicated by the black box. Adopted and modified according Chen et al 25.
1. Chen, R. W.; Aaltonen, L. M.; Vaheri, A., Human papillomavirus type 16 in head and neck carcinogenesis. Reviews in Medical Virology 2005, 15, 351-363.
2. Syrjanen, S., Human papillomavirus (HPV) in head and neck cancer. Journal of Clinical Virology 2005, 32, S59-S66.
3. Badulescu, F.; Crisan, A.; Badulescu, A.; Schenker, M., Recent data about the role of human papillomavirus (HPV) in oncogenesis of head and neck cancer. Romanian Journal of Morphology and Embryology 2010, 51, 437-440.
4. Fakhry, C.; Westra, W. H.; Cmelak, S. L. A.; Ridge, J. A.; Pinto, H.; Forastiere, A.; Gillison, M. L., Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. Journal of the National Cancer Institute 2008, 100, 261-269.
5. Rose, B. R.; Thompson, C. H.; Tattersall, M. H.; O'Brien, C. J.; Cossart, Y. E., Squamous carcinoma of the head and neck: Molecular mechanisms and potential biomarkers. Australian and New Zealand Journal of Surgery 2000, 70, 601-606.
6. Yuan, C. H.; Filippova, M.; Duerksen-Hughes, P., Modulation of Apoptotic Pathways by Human Papillomaviruses (HPV): Mechanisms and Implications for Therapy. Viruses-Basel 2012, 4, 3831-3850.
7. Smith, E. M.; Ritchie, J. M.; Pawlita, M.; Rubenstein, L. M.; Haugen, T. H.; Turek, L. P.; Hamsikova, E., Human papillomavirus seropositivity and risks of head and neck cancer. International Journal of Cancer 2007, 120, 825-832.
8. Janicek, M. F.; Averette, H. E., Cervical cancer: Prevention, diagnosis, and therapeutics. Ca-a Cancer Journal for Clinicians 2001, 51, 92-114.
9. Doorbar, J.; Gallimore, P. H., Identification of proteins encoded by the L1 and L2 open reading frames of human papillomavirus-1A Journal of Virology 1987, 61, 2793-2799.
10. Syrjanen, S., Human papillomavirus infections and oral tumors. Medical Microbiology and Immunology 2003, 192, 123-128.
11. Syrjanen, K.; Syrjanen, S., Detection of human papillomavirus in sinonasal papillomas: Systematic review and meta-analysis. Laryngoscope 2013, 123, 181-192.
12. van Houten, V. M. M.; Leemans, C. R.; Kummer, J. A.; Dijkstra, J.; Kuik, D. J.; van den Brekel, M. W. M.; Snow, G. B.; Brakenhoff, R. H., Molecular diagnosis of surgical margins and local recurrence in head and neck cancer patients: A prospective study. Clinical Cancer Research 2004, 10, 3614-3620.
13. zur Hausen, H., Human papillomavirus & cervical cancer. Indian Journal of Medical Research 2009, 130, 209-209.
14. Foguel, D.; Silva, J. L.; de Prat-Gay, G., Characterization of a partially folded monomer of the DNA-binding domain of human papillomavirus E2 protein obtained at high pressure. Journal of Biological Chemistry 1998, 273, 9050-9057.
15. Ustav, M.; Ustav, E.; Szymanski, P.; Stenlund, A., Identification of the origin of replication of bovine papillomavirus and characterization of the viral origin recognition factor-E1 Embo Journal 1991, 10, 4321-4329.
16. Garnett, T. O.; Duerksen-Hughes, P. J., Modulation of apoptosis by human papillomavirus (HPV) oncoproteins. Archives of Virology 2006, 151, 2321-2335.
17. Dayyani, F.; Etzel, C. J.; Liu, M.; Ho, C. H.; Lippman, S. M.; Tsao, A. S., Meta-analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC). Head & Neck Oncology 2010, 2.
18. Strati, K.; Pitot, H. C.; Lambert, P. F., Identification of biomarkers that distinguish human papillomavirus (HPV)-positive versus HPV-negative head and neck cancers in a mouse model. Proceedings of the National Academy of Sciences of the United States of America 2006, 103, 14152-14157.
19. Van Doorslaer, K.; Sidi, A.; Zanier, K.; Rybin, V.; Deryckere, F.; Rector, A.; Burk, R. D.; Lienau, E. K.; van Ranst, M.; Trave, G., Identification of Unusual E6 and E7 Proteins within Avian Papillomaviruses: Cellular Localization, Biophysical Characterization, and Phylogenetic Analysis. Journal of Virology 2009, 83, 8759-8770.
20. Wiest, T.; Schwarz, E.; Enders, C.; Flechtenmacher, C.; Bosch, F. X., Involvement of intact HPV16 E6/E7 gene expression in head and neck cancers with unaltered p53 status and perturbed pRb cell cycle control. Oncogene 2002, 21, 1510-1517.
21. Zimmermann, H.; Degenkolbe, R.; Bernard, H. U.; O'Connor, M. J., The human papillomavirus type 16 E6 oncoprotein can down-regulate p53 activity by targeting the transcriptional coactivator CBP/p300. Journal of Virology 1999, 73, 6209-6219.
22. Dyson, N.; Howley, P. M.; Munger, K.; Harlow, E., The human papilloma virus-16 E7-oncoprotein is able to bind to the retinoblastoma gene-product Science 1989, 243, 934-937.
23. Smith, E. M.; Pawlita, M.; Rubenstein, L. M.; Haugen, T. H.; Hamsikova, E.; Turek, L. P., Risk factors and survival by HPV-16 E6 and E7 antibody status in human papillomavirus positive head and neck cancer. International Journal of Cancer 2010, 127, 111-117.
24. Syrjanen, S.; Rautava, J., HPV and oral health response Journal of the American Dental Association 2012, 143, 442-444.
25. Chen, A. Y.; DeSantis, C.; Jemal, A., US Mortality Rates for Oral Cavity and Pharyngeal Cancer by Educational Attainment. Archives of Otolaryngology-Head & Neck Surgery 2011, 137, 1094-1099.
26. Venuti, A.; Paolini, F., HPV detection methods in head and neck cancer. Head and neck pathology 2012, 6 Suppl 1, S63-74.
27. Gagnon, D.; Fradet-Turcotte, A.; Archambault, J., A quantitative and high-throughput assay of human papillomavirus DNA replication. Methods in molecular biology (Clifton, N.J.) 2015, 1249, 305-316.
28. Moreas, H.; Tsiambas, E.; Lazaris, A. C.; Nonni, A.; Karameris, A.; Metaxas, G. E.; Armatas, H. E.; Patsouris, E., Impact of HPV detection in colorectal adenocarcinoma: HPV protein and chromogenic in situ hybridization analysis based on tissue microarrays. Journal of Buon 2014, 19, 91-96.
29. Haugg, A. M.; Rennspiess, D.; zur Hausen, A.; Speel, E. J. M.; Cathomas, G.; Becker, J. C.; Schrama, D., Fluorescence in situ hybridization and qPCR to detect Merkel cell polyomavirus physical status and load in Merkel cell carcinomas. International Journal of Cancer 2014, 135, 2804-2815.
30. Rodel, F.; Wieland, U.; Fraunholz, I.; Kitz, J.; Rave-Frank, M.; Wolff, H. A.; Weiss, C.; Wirtz, R.; Balermpas, P.; Fokas, E.; Rodel, C., Human papillomavirus DNA load and p16(INK4a) expression predict for local control in patients with anal squamous cell carcinoma treated with chemoradiotherapy. International journal of cancer. Journal international du cancer 2015, 136, 278-288.
31. Heidegger, I.; Pichler, R.; Muller, B.; Klocker, H.; Oswald, D.; Haid, B.; Zelger, B.; Horninger, W.; Oswald, J., Is real-time PCR the correct method to evaluate the incidence of human papillomavirus in prepuces of asymptomatic boys and men? World Journal of Urology 2014, 32, 1199-1204.
32. Micalessi, M. I.; Boulet, G. A.; Bogers, J., A Real-Time PCR Approach Based on SPF10 Primers and the INNO-LiPA HPV Genotyping Extra Assay for the Detection and Typing of Human Papillomavirus. Methods in molecular biology (Clifton, N.J.) 2015, 1249, 27-35.
33. Sahiner, F.; Kubar, A.; Gumral, R.; Ardic, M.; Yigit, N.; Sener, K.; Dede, M.; Yapar, M., Efficiency of MY09/11 consensus PCR in the detection of multiple HPV infections. Diagnostic Microbiology and Infectious Disease 2014, 80, 43-49.
34. Vega-Pena, A.; Illades-Aguiar, B.; Flores-Alfaro, E.; Lopez-Bayghen, E.; Reyes-Maldonado, E.; Alarcon-Romero, L. D., Correlation between KI-67 and telomerase expression with in situ hybridization for high-risk human papillomavirus Archives of Biological Sciences 2013, 65, 81-90.
35. Singhi, A. D.; Westra, W. H., Comparison of Human Papillomavirus In Situ Hybridization and p16 Immunohistochemistry in the Detection of Human Papillomavirus-Associated Head and Neck Cancer Based on a Prospective Clinical Experience. Cancer 2010, 116, 2166-2173.
36. Salehinejad, J.; Sharifi, N.; Amirchaghmaghi, M.; Ghazi, N.; Shakeri, M. T.; Ghazi, A., Immunohistochemical expression of p16 protein in oral squamous cell carcinoma and lichen planus. Annals of Diagnostic Pathology 2014, 18, 210-213.
37. Shimizu, A.; Kato, M.; Takeuchi, Y.; Sano, T.; Kaira, K.; Uezato, H.; Ishikawa, O., Detection of human papillomavirus (HPV) in patients with squamous cell carcinoma and the clinical characteristics of HPV-positive cases. The British journal of dermatology 2014, 171, 779-785.
J.Met.Nano:
volume-1, issue-4
- Human papilloma virus (HPV) and methods for its identification in head and neck cancers
- Clinical application of capillary electrophoresis – determination of free amino acids in body fluids
- The role of phytochelatins in plant and animals: A review
- Paramagnetic particles for immobilization of metallothionein – promising biomarker of head and neck cancer
- Delivery of doxorubicin using protein nanocarriers
- MIR-150 electrochemical detection connected with specific isolation based on magnetic particles
- Nanomaghemite core functionalized with ion-exchange resins for isolation of biogennic amines
- Modern techniques of increase the antibacterial properties of the instruments
- Detection of sentinel lymph node using magnetic nanoparticles
- Opening of working and meeting room for nanobiometalnet project
- New directions of electrochemistry, bioelectrochemistry,nanoelectrochemistry and bioengineering
 PDF
PDF