
Influence of different forms of glutathione on Lactobacillus casei ssp. rhamnosus culture
Zuzana Lackova, Marketa Kominkova, Ondrej Zitka, Rene Kizek
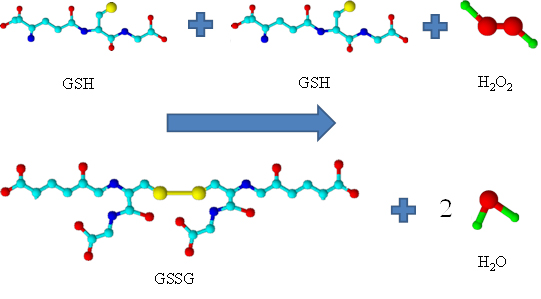
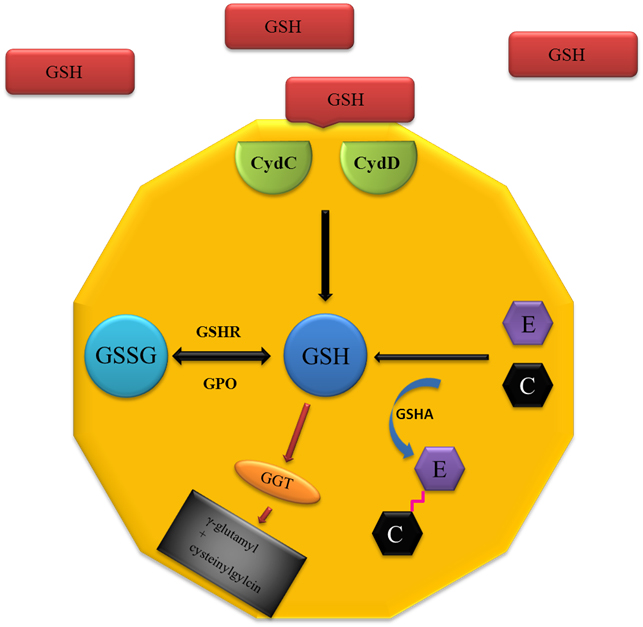
Influence of different forms of glutathione on Lactobacillus casei ssp. rhamnosus culture. Lactobacillus casei ssp. rhamnosus (L. rhamnosus) of the genus Lactobacillus belongs to the facultatively heterofermentative lactic acid bacteria. In the human body it occurs mainly in the intestine, where it brings a beneficial effect on the immune system. Further, the antioxidant properties and action against oxidative stress belong among its major advantages. In the genus Lactobacillus also the ability to reduce clinical symptoms of influenza was demonstrated. Similar properties to that of the genus Lactobacillus are demonstrated also in glutathione (γ-glutamylcysteinylglycin), one of the most important thiols present in both plant and animal tissues, and also in number of microorganisms. Due to the wide field of application of bacteria of the genus Lactobacillus and possibilities to improve some of their properties in the presence of glutathione, the use of lactobacilli with the addition of glutathione can be considered for the treatment of influenza and support the treatment of influenza illness.
1. Sedláček I., Taxonomie prokaryot, Masarykova univerzita, 2007.
2. Collins M. D., Phillips B. A., Zanoni P.: International Journal of Systematic Bacteriology, 39, 105 (1989).
3. Holzapfel W. H., Haberer P., Geisen R., Bjorkroth J., Schillinger U.: American Journal of Clinical Nutrition, 73, 365S (2001).
4. Kamaladevi A., Ganguli A., Kumar M., Balamurugan K.: Pesticide Biochemistry and Physiology, 105, 213 (2013).
5. Masip L., Veeravalli K., Georgioui G.: Antioxidants & Redox Signaling, 8, 753 (2006).
6. Broniowska K. A., Diers A. R., Hogg N.: Biochimica Et Biophysica Acta-General Subjects, 1830, 3173 (2013).
7. Stiles M. E., Holzapfel W. H.: International Journal of Food Microbiology, 36, 1 (1997).
8. Li Y., Hugenholtz J., Abee T., Molenaar D.: Applied and Environmental Microbiology, 69, 5739 (2003).
9. Knejzlik Z., Kas J., Ruml T.: Chemicke Listy, 94, 913 (2000).
10. Bernardeau M., Guguen M., Vernoux J. P.: FEMS Microbiology Reviews, 30, 487 (2006).
11. Peran L., Camuesco D., Comalada M., Bailon E., Henriksson A., Xaus J., Zarzuelo A., Galvez J.: Journal of Applied Microbiology, 103, 836 (2007).
12. Waki N., Yajima N., Suganuma H., Buddle B. M., Luo D., Heiser A., Zheng T.: Letters in Applied Microbiology, 58, 87 (2014).
13. Park M. K., Ngo V., Kwon Y. M., Lee Y. T., Yoo S., Cho Y. H., Hong S. M., Hwang H. S., Ko E. J., Jung Y. J., Moon D. W., Jeong E. J., Kim M. C., Lee Y. N., Jang J. H., Oh J. S., Kim C. H., Kang S. M.: Plos One, 8, (2013).
14. Kiso M., Takano R., Sakabe S., Katsura H., Shinya K., Uraki R., Watanabe S., Saito H., Toba M., Kohda N., Kawaoka Y.: Scientific Reports, 3, (2013).
15. Hori T., Kiyoshima J., Shida K., Yasui H.: Clinical and Diagnostic Laboratory Immunology, 8, 593 (2001).
16. Klaban V., Ekologie mikroorganismů: ilustrovaný lexikon biologie, ekologie a patogenity mikroorganismů, Galén, 2011.
17. Salminen S. J., Gueimonde M., Isolauri E.: Journal of Nutrition, 135, 1294 (2005).
18. Klaenhammer T. R., Kullen M. J.: International Journal of Food Microbiology, 50, 45 (1999).
19. Pophaly S. D., Singh R., Kaushik J. K., Tomar S. K.: Microbial Cell Factories, 11, (2012).
20. Kullisaar T., Songisepp E., Aunapuu M., Kilk K., Arend A., Mikelsaar M., Rehema A., Zilmer M.: Applied Biochemistry and Microbiology, 46, 481 (2010).
21. Kubienova L., Ticha T., Jahnova J., Luhova L., Petrivalsky M.: Chemicke Listy, 107, 202 (2013).
J.Met.Nano:
volume-1, issue-1
- Liposomal transporters
- Modern imaging techniques
- General Mechanisms of Microbial resistance to metals
- MiRNA: From biogenesis to medical utilization
- Prion proteins and its interaction with heavy metals
- Monitoring of the role of metal ions and Metallothionein in the prion diseases development
- Viral drug nanocarriers
- Applications of micro-flow systems
- Biosynthesis of quantum dots
- Influence of different forms of glutathione on Lactobacillus casei ssp. rhamnosus culture
- Green synthesis of silver and gold nanoparticles with extracts from higher plants
- Apoferritin: nanocarrier for targeted drug delivery
- Nucleic acids detection by dot-blot method
 PDF
PDF