
The ratio of GSH/GSSG in biological organisms
Marketa Kominkova, Ondrej Zitka, Rene Kizek
Tripeptide glutathion is one of the most important thiol compound. It is involved in a wide variety of cellular processes. In the organism it occurs in two forms: as a reduced glutathion (GSH) and as an oxidized glutathion (GSSG). Protective and regulatory functions of GSH are based on changes in its redox state. GSH and GSSG forms together one of the most significant redox couples in the cell. Their ratio under the physiological conditions is usually constant, regardless of the total concentration of glutathion in a cell. The ratio of both forms of glutathion is considered as an indicator of oxidative stress effect.
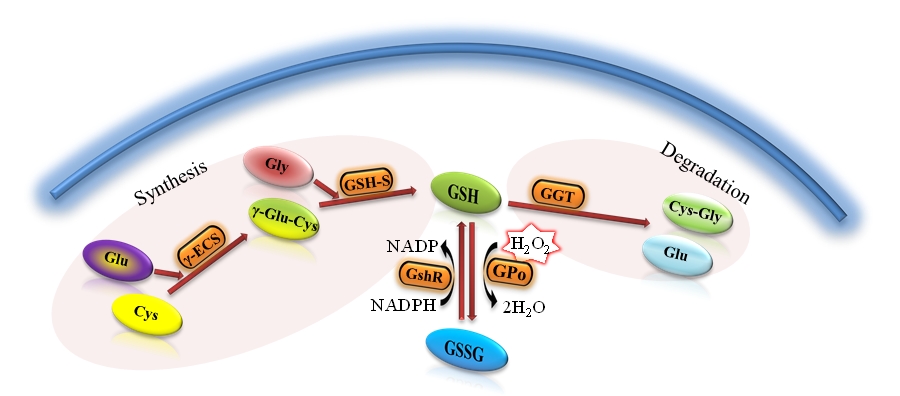
Fig. 1: The figure shows the general procedure for the synthesis and metabolism of glutathione. From glutamic acid (Glu) and cysteine (Cys) is created by the enzyme γ-synthase glutamylcystein (γ-ECS) γ-glutamylcystein (γ-Glu - Cys). The effect of the enzyme glutathione synthase (GSH-S) from γ-Glu-Cys and glycine (Gly) synthesized glutathione reduced form (GSH). As an antioxidant, GSH is oxidized to form oxidized glutathione (GSSG) with the participation of the enzyme glutathione peroxidase (GPO). Thanks to the action of glutathione reductase (GshR) glutathione occurs mainly in the form of GSH. Degradation of glutathione in cells is due to the γ-glutamyl transpeptidase (GGT) for glutamic acid (Glu) and dipeptide cysteinylglycine (Cys-Gly)
1. De Rey-Pailhade, J. Sur un corps d’origine organique hydrogénant le soufre 1 à froid. Comptes Rendus Hebdomadaire Séances de l’Académie de Sciences. 1888, 1683–1684.
2. Meister, A. On the Biochemistry of Glutathione, Glutathione Centennial, 1989, pp. 3-21.
3. Pophaly, S.D.; Singh, R.; Kaushik, J.K.; Tomar, S.K. Current status and emerging role of glutathione in food grade lactic acid bacteria. Microbial Cell Factories. 2012, 11.
4. Seth, C.S.; Remans, T.; Keunen, E.; Jozefczak, M.; Gielen, H.; Opdenakker, K.; Weyens, N.; Vangronsveld, J.; Cuypers, A. Phytoextraction of toxic metals: a central role for glutathione. Plant Cell and Environment. 2012, 35, 334-346.
5. Paradiso, A.; Berardino, R.; de Pinto, M.C.; di Toppi, L.S.; Storelli, M.M.; Tommasi, F.; De Gara, L. Increase in ascorbate-glutathione metabolism as local and precocious systemic responses induced by cadmium in durum wheat plants. Plant and Cell Physiology. 2008, 49, 362-374.
6. Forman, H.J.; Zhang, H.Q.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Molecular Aspects of Medicine. 2009, 30, 1-12.
7. Pastore, A.; Federici, G.; Bertini, E.; Piemonte, F. Analysis of glutathione: implication in redox and detoxification. Clinica Chimica Acta. 2003, 333, 19-39.
8. Biswas, S.K.; Rahman, I. Environmental toxicity, redox signaling and lung inflammation: The role of glutathione. Molecular Aspects of Medicine. 2009, 30, 60-76.
9. Anderson, M.E. Glutathione: an overview of biosynthesis and modulation. Chemico-Biological Interactions. 1998, 112, 1-14.
10. Ogawa, K. Glutathione-associated regulation of plant growth and stress responses. Antioxidants & Redox Signaling. 2005, 7, 973-981.
11. Szalai, G.; Kellos, T.; Galiba, G.; Kocsy, G. Glutathione as an Antioxidant and Regulatory Molecule in Plants Under Abiotic Stress Conditions. Journal of Plant Growth Regulation. 2009, 28, 66-80.
12. Noctor, G.; Veljovic-Jovanovic, S.; Driscoll, S.; Novitskaya, L.; Foyer, C.H. Drought and oxidative load in the leaves of C3 plants: a predominant role for photorespiration? Ann Bot. 2002, 89 Spec No, 841-850.
13. Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. International Journal of Biochemistry & Cell Biology. 2007, 39, 44-84.
J.Met.Nano:
volume-1, issue-3
- Personal and professional representation of the nanolabsys project
- Administration and information system of the project
- Microwave preparation of carbon quantum dots with different surface modification
- Cell lines as a model system for quantum dots applications
- Application of quantum dots into chicken embryos
- The influence of zinc to living organisms
- The influence of cadmium to living organisms
- The influence of lead to living organisms
- The influence of mercury to living organisms
- Monitoring of metallothionein levels in biological organism exposed to the metal elements and compounds
- The ratio of GSH/GSSG in biological organisms
- Amino Acids and their interactions with heavy metals
- Antioxidat enzymes – biochemical markers of oxidative stress
- Study of the interaction of quantum dots with tumor cells by fluorescence microscopy
- Flow-cytometric analysis of programmed cell death
- Bacteriophage λ as a doxorubicin nanocarrier
 PDF
PDF