
Antioxidat enzymes – biochemical markers of oxidative stress
Martina Matouskova, Branislav Ruttkay-Nedecky,Rene Kizek
Oxidative stress is the imbalance between the oxygen radicals and antioxidant defence system of the organism and leads to cell damage. Antioxidant enzymes are an important part of the defense against oxidative stress and the crucial ones are superoxide dismutase, catalase, glutathione peroxidase and glutathione reductase. Superoxide dismutase catalyses the conversion of superoxide to hydrogen peroxide, which can be further degraded by catalase to oxygen and water. Peroxidases are enzymes catalysing the reduction of the number of peroxides to alcohols. Glutathion peroxidases are selenium dependent enzymes using reduced glutathione (GSH) as a cofactor. They catalyze the conversion of hydrogen peroxide to water while the reduced glutathione is oxidized. The GSH is then being renewed by the activity of glutathione reductase, another antioxidative enzyme that reduces oxidized glutathione (GSSG) to GSH.
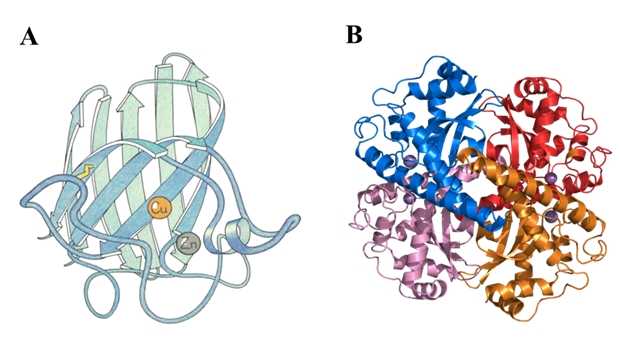
Fig.1: A) subunit structure of Cu-Zn SOD1 isolated from bovine erythrocytes [3]; B) the structure of human mitochondrial SOD2 Mn [4]
1. Paoletti, F.; Aldinucci, D.; Mocali, A.; Caparrini, A. A SENSITIVE SPECTROPHOTOMETRIC METHOD FOR THE DETERMINATION OF SUPEROXIDE-DISMUTASE ACTIVITY IN TISSUE-EXTRACTS. Analytical Biochemistry. 1986, 154, 536-541.
2. Skalicka, Z.F.; Zolzer, F.; Beranek, L.; Racek, J. Indicators of oxidative stress after ionizing and/or non-ionizing radiation: Superoxid dismutase and malondialdehyde. Journal of Photochemistry and Photobiology B-Biology. 2012, 117, 111-114.
3. Tainer, J.A.; Getzoff, E.D.; Beem, K.M.; Richardson, J.S.; Richardson, D.C. DETERMINATION AND ANALYSIS OF THE 2A STRUCTURE OF COPPER, ZINC SUPEROXIDE-DISMUTASE. Journal of Molecular Biology. 1982, 160, 181-217.
4. Borgstahl, G.E.O.; Parge, H.E.; Hickey, M.J.; Johnson, M.J.; Boissinot, M.; Hallewell, R.A.; Lepock, J.R.; Cabelli, D.E.; Tainer, J.A. Human mitochondrial manganese superoxide dismutase polymorphic variant Ile58Thr reduces activity by destabilizing the tetrameric interface. Biochemistry. 1996, 35, 4287-4297.
5. Paoletti, F.; Mocali, A. DETERMINATION OF SUPEROXIDE-DISMUTASE ACTIVITY BY PURELY CHEMICAL-SYSTEM BASED ON NAD(P)H OXIDATION. Methods in Enzymology. 1990, 186, 209-220.
6. Tarhan, L.; Tuzmen, M.N. Some properties of Cu, Zn-superoxide dismutase from sheep erythrocyte. Turkish Journal of Chemistry. 2000, 24, 109-116.
7. Flohe, L.; Gunzler, W.A. ASSAYS OF GLUTATHIONE-PEROXIDASE. Methods in Enzymology. 1984, 105, 114-121.
8. Chance, B. THE EFFECT OF PH UPON THE EQUILIBRIA OF CATALASE COMPOUNDS. Journal of Biological Chemistry. 1952, 194, 483-496.
9. Barr, D.P.; Aust, S.D. ON THE MECHANISM OF PEROXIDASE-CATALYZED OXYGEN PRODUCTION. Archives of Biochemistry and Biophysics. 1993, 303, 377-382.
10. Masters, C.; Crane, D. The peroxisome: A vital organelle, Cambridge University Press, The Pitt Building, Trumpington Street, Cambridge CB2 1RP, England; Cambridge University Press, 40 W. 20th Street, New York, New York 10011-4211, USA, 1995.
11. McCammon, M.T.; Veenhuis, M.; Trapp, S.B.; Goodman, J.M. ASSOCIATION OF GLYOXYLATE AND BETA-OXIDATION ENZYMES WITH PEROXISOMES OF SACCHAROMYCES-CEREVISIAE. Journal of Bacteriology. 1990, 172, 5816-5827.
12. Binder, M.; Schanz, M.; Hartig, A. VECTOR-MEDIATED OVEREXPRESSION OF CATALASE-A IN THE YEAST SACCHAROMYCES CEREVISIAE INDUCES INCLUSION BODY FORMATION. European Journal of Cell Biology. 1991, 54, 305-312.
13. Schellhorn, H.E. REGULATION OF HYDROPEROXIDASE (CATALASE) EXPRESSION IN ESCHERICHIA-COLI. Fems Microbiology Letters. 1995, 131, 113-119.
14. Fridovich, I. SUPEROXIDE RADICAL AND SUPEROXIDE DISMUTASES. Annual Review of Biochemistry. 1995, 64, 97-112.
15. Aebi, H. CATALASE INVITRO. Methods in Enzymology. 1984, 105, 121-126.
16. Leonel, C.; Gelaleti, G.B.; Jardim, B.V.; Moschetta, M.G.; Regiani, V.R.; Oliveira, J.G.; Zuccari, D. Expression of glutathione, glutathione peroxidase and glutathione S-transferase pi in canine mammary tumors. Bmc Veterinary Research. 2014, 10.
17. van der Oost, R.; Beyer, J.; Vermeulen, N.P.E. Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environmental Toxicology and Pharmacology. 2003, 13, 57-149.
18. Arthur, J.R.; Bermano, G.; Mitchell, J.H.; Hesketh, J.E. Regulation of selenoprotein gene expression and thyroid hormone metabolism. Biochemical Society Transactions. 1996, 24, 384-388.
19. Burk, R.F.; Hill, K.E.; Motley, A.K. Selenoprotein metabolism and function: Evidence for more than one function for selenoprotein P. Journal of Nutrition. 2003, 133, 1517S-1520S.
20. Carlberg, I.; Mannervik, B. PURIFICATION AND CHARACTERIZATION OF FLAVOENZYME GLUTATHIONE REDUCTASE FROM RAT-LIVER. Journal of Biological Chemistry. 1975, 250, 5475-5480.
J.Met.Nano:
volume-1, issue-3
- Personal and professional representation of the nanolabsys project
- Administration and information system of the project
- Microwave preparation of carbon quantum dots with different surface modification
- Cell lines as a model system for quantum dots applications
- Application of quantum dots into chicken embryos
- The influence of zinc to living organisms
- The influence of cadmium to living organisms
- The influence of lead to living organisms
- The influence of mercury to living organisms
- Monitoring of metallothionein levels in biological organism exposed to the metal elements and compounds
- The ratio of GSH/GSSG in biological organisms
- Amino Acids and their interactions with heavy metals
- Antioxidat enzymes – biochemical markers of oxidative stress
- Study of the interaction of quantum dots with tumor cells by fluorescence microscopy
- Flow-cytometric analysis of programmed cell death
- Bacteriophage λ as a doxorubicin nanocarrier
 PDF
PDF