

Dobrá díla jsou plodem dobrého charakteru a protože je chvályhodnější příčina než následek, chval více dobrý charakter bez vzdělání než vzdělance bez charakteru.
Leonardo da Vinci
Výzkum
Nanocarriers for anticancer drugs – New trends in nanomedicine
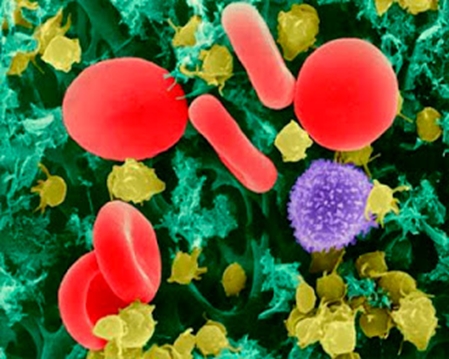 Nanomedicine is the newest member of branches in molecular nanotechnology. It serves for monitoring, repairing, building, and control of biological systems on molecular level, which is carried out by nanocomponents and nanosystems (Fig. 1). While molecular nanotechnology operates within the range from hundredths to thousandths nanometers, the basic structural element of molecular nanotechnology is an atom with diameter one nm [1-4]. Dramatic advancement of these technologies can be expected, especially in diagnostics of diseases in their early stages [5-9]. Nowadays, we can divide nanomedicinal approaches into two sections: i) currently in used and ii) real future perspectives. In the approaches with real future perspectives, we may see therapeutic abilities of nanorobots in microsurgery and in treatment of various types of diseases, such as coronary thrombosis or malignant tumors. We can expect that medicinal nanorobots with size up to 1,000 nm can be injected into human body (some milliards of nanorobots correspond to one milliliter). There they can help to immune system, participate in processes of metabolism, perform repairing operations, eventually cluster together into higher structures and form more complicated and effective repairing and protective systems. Bio-implants in the field of skin regeneration (special polymers, silver nanoparticles) are very important nanomedicinal group, which is currently used.
Nanodelivering of a drug belongs to other nanomedicinal group which gained the great attention of researchers. The development of each new drug has basically two main aspects – maximal effectiveness against the existing disease and as minimal side effects (effect on non-target tissue) as possible (Fig. 2). However, some difficulties connected with the drug delivery may occur, such as troublesome solubility and biological availability, short time of circulation in blood channels, inconvenient biodistribution to the target organ. Nanoparticle-mediated targeted delivery of drugs might significantly reduce the dosage of the drugs, optimized drug loading and release properties, ,increase its specificity and bioavailability including shelf-life and reduce the toxicity [10-12]. These advantages of nanoparticles compared to convenient small molecules for chemotherapy make them promising candidate to combat cancer drug resistance [13]. From the point of view of diseases, in cancer therapy, targeted delivery in a localized way is one of the key challenges. Tumor targeting with nanoparticles can be realized through passive and active way [12, 14-16]. The particles can be modified with various types of materials including biomolecules (Fig. 3). Using various organizations of atoms, resulting properties of particular material, such as elasticity, plasticity, strength, or conductivity can be modified.
The first mentioned improvement in cancer therapy is based on the enhanced permeability and retention (EPR) effect of the vasculature surrounding tumors. The active way relies on ligand-directed binding of nanoparticles to receptors expressed by tumor cells [17]. These ligands comprise antibodies, peptides, nucleic acid aptamers, carbohydrates and small molecules [11]. The key features of anticancer nanoparticles are mainly nanoparticles size, surface properties (e.g. hydrophobicity) and targeting ligands (Fig. 3). Generally, 200 nm is considered as upper limit for nanoparticles size, while the minimal diameter should be about 10 nm. Certainly, nanoparticles properties requirements also depend on tumor characteristics including cancer type, stage of disease, site in the body and host species, which is summarized in review of Adiseshaiah et al. [18].
Nanoparticles designed for tumor targeted therapies consist of various components, in most cases from nanocarrier and an active agent (drug) [19]. Drug-carrier nanoparticles are considered as submicroscopic colloidal systems that may act as drug vehicles, either as nanospheres (matrix system in which the drug is dispersed) or nanocapsules (reservoirs in which the drug is confined in hydrophobic or hydrophilic cor Nanomedicine is the newest member of branches in molecular nanotechnology. It serves for monitoring, repairing, building, and control of biological systems on molecular level, which is carried out by nanocomponents and nanosystems (Fig. 1). While molecular nanotechnology operates within the range from hundredths to thousandths nanometers, the basic structural element of molecular nanotechnology is an atom with diameter one nm [1-4]. Dramatic advancement of these technologies can be expected, especially in diagnostics of diseases in their early stages [5-9]. Nowadays, we can divide nanomedicinal approaches into two sections: i) currently in used and ii) real future perspectives. In the approaches with real future perspectives, we may see therapeutic abilities of nanorobots in microsurgery and in treatment of various types of diseases, such as coronary thrombosis or malignant tumors. We can expect that medicinal nanorobots with size up to 1,000 nm can be injected into human body (some milliards of nanorobots correspond to one milliliter). There they can help to immune system, participate in processes of metabolism, perform repairing operations, eventually cluster together into higher structures and form more complicated and effective repairing and protective systems. Bio-implants in the field of skin regeneration (special polymers, silver nanoparticles) are very important nanomedicinal group, which is currently used.
Nanodelivering of a drug belongs to other nanomedicinal group which gained the great attention of researchers. The development of each new drug has basically two main aspects – maximal effectiveness against the existing disease and as minimal side effects (effect on non-target tissue) as possible (Fig. 2). However, some difficulties connected with the drug delivery may occur, such as troublesome solubility and biological availability, short time of circulation in blood channels, inconvenient biodistribution to the target organ. Nanoparticle-mediated targeted delivery of drugs might significantly reduce the dosage of the drugs, optimized drug loading and release properties, ,increase its specificity and bioavailability including shelf-life and reduce the toxicity [10-12]. These advantages of nanoparticles compared to convenient small molecules for chemotherapy make them promising candidate to combat cancer drug resistance [13]. From the point of view of diseases, in cancer therapy, targeted delivery in a localized way is one of the key challenges. Tumor targeting with nanoparticles can be realized through passive and active way [12, 14-16]. The particles can be modified with various types of materials including biomolecules (Fig. 3). Using various organizations of atoms, resulting properties of particular material, such as elasticity, plasticity, strength, or conductivity can be modified.
The first mentioned improvement in cancer therapy is based on the enhanced permeability and retention (EPR) effect of the vasculature surrounding tumors. The active way relies on ligand-directed binding of nanoparticles to receptors expressed by tumor cells [17]. These ligands comprise antibodies, peptides, nucleic acid aptamers, carbohydrates and small molecules [11]. The key features of anticancer nanoparticles are mainly nanoparticles size, surface properties (e.g. hydrophobicity) and targeting ligands (Fig. 3). Generally, 200 nm is considered as upper limit for nanoparticles size, while the minimal diameter should be about 10 nm. Certainly, nanoparticles properties requirements also depend on tumor characteristics including cancer type, stage of disease, site in the body and host species, which is summarized in review of Adiseshaiah et al. [18].
Nanoparticles designed for tumor targeted therapies consist of various components, in most cases from nanocarrier and an active agent (drug) [19]. Drug-carrier nanoparticles are considered as submicroscopic colloidal systems that may act as drug vehicles, either as nanospheres (matrix system in which the drug is dispersed) or nanocapsules (reservoirs in which the drug is confined in hydrophobic or hydrophilic core surrounded by a single polymeric membrane) [20]. Nanoparticle carriers are mostly composed of iron oxides, gold, biodegradable polymers, dendrimers, lipid based carriers such as liposomes and micelles, viruses (viral nanoparticles) and even organometallic compound [11, 21, 22]. A detailed review about nanocariers was recently published by Peer et al [23]. The drug encapsulation in nanocarrier provides better biocompatibility and hence its potential use in clinical oncology. Several such engineered drugs are already in clinical practice, including liposomal doxorubicin and albumin conjugate paclitaxel [24]. However, the potential success of these particles in the clinic relies on consideration of above mentioned important parameters, but most importantly, minimum toxicity of the carrier itself [25]. Concerning the nanoparticles shape, following nanostructures are frequently cited in literature: nanoshells, nanorods, nanocages, nanocubes or nanotubes.
Nanomedicine is the newest member of branches in molecular nanotechnology. It serves for monitoring, repairing, building, and control of biological systems on molecular level, which is carried out by nanocomponents and nanosystems (Fig. 1). While molecular nanotechnology operates within the range from hundredths to thousandths nanometers, the basic structural element of molecular nanotechnology is an atom with diameter one nm [1-4]. Dramatic advancement of these technologies can be expected, especially in diagnostics of diseases in their early stages [5-9]. Nowadays, we can divide nanomedicinal approaches into two sections: i) currently in used and ii) real future perspectives. In the approaches with real future perspectives, we may see therapeutic abilities of nanorobots in microsurgery and in treatment of various types of diseases, such as coronary thrombosis or malignant tumors. We can expect that medicinal nanorobots with size up to 1,000 nm can be injected into human body (some milliards of nanorobots correspond to one milliliter). There they can help to immune system, participate in processes of metabolism, perform repairing operations, eventually cluster together into higher structures and form more complicated and effective repairing and protective systems. Bio-implants in the field of skin regeneration (special polymers, silver nanoparticles) are very important nanomedicinal group, which is currently used.
Nanodelivering of a drug belongs to other nanomedicinal group which gained the great attention of researchers. The development of each new drug has basically two main aspects – maximal effectiveness against the existing disease and as minimal side effects (effect on non-target tissue) as possible (Fig. 2). However, some difficulties connected with the drug delivery may occur, such as troublesome solubility and biological availability, short time of circulation in blood channels, inconvenient biodistribution to the target organ. Nanoparticle-mediated targeted delivery of drugs might significantly reduce the dosage of the drugs, optimized drug loading and release properties, ,increase its specificity and bioavailability including shelf-life and reduce the toxicity [10-12]. These advantages of nanoparticles compared to convenient small molecules for chemotherapy make them promising candidate to combat cancer drug resistance [13]. From the point of view of diseases, in cancer therapy, targeted delivery in a localized way is one of the key challenges. Tumor targeting with nanoparticles can be realized through passive and active way [12, 14-16]. The particles can be modified with various types of materials including biomolecules (Fig. 3). Using various organizations of atoms, resulting properties of particular material, such as elasticity, plasticity, strength, or conductivity can be modified.
The first mentioned improvement in cancer therapy is based on the enhanced permeability and retention (EPR) effect of the vasculature surrounding tumors. The active way relies on ligand-directed binding of nanoparticles to receptors expressed by tumor cells [17]. These ligands comprise antibodies, peptides, nucleic acid aptamers, carbohydrates and small molecules [11]. The key features of anticancer nanoparticles are mainly nanoparticles size, surface properties (e.g. hydrophobicity) and targeting ligands (Fig. 3). Generally, 200 nm is considered as upper limit for nanoparticles size, while the minimal diameter should be about 10 nm. Certainly, nanoparticles properties requirements also depend on tumor characteristics including cancer type, stage of disease, site in the body and host species, which is summarized in review of Adiseshaiah et al. [18].
Nanoparticles designed for tumor targeted therapies consist of various components, in most cases from nanocarrier and an active agent (drug) [19]. Drug-carrier nanoparticles are considered as submicroscopic colloidal systems that may act as drug vehicles, either as nanospheres (matrix system in which the drug is dispersed) or nanocapsules (reservoirs in which the drug is confined in hydrophobic or hydrophilic cor Nanomedicine is the newest member of branches in molecular nanotechnology. It serves for monitoring, repairing, building, and control of biological systems on molecular level, which is carried out by nanocomponents and nanosystems (Fig. 1). While molecular nanotechnology operates within the range from hundredths to thousandths nanometers, the basic structural element of molecular nanotechnology is an atom with diameter one nm [1-4]. Dramatic advancement of these technologies can be expected, especially in diagnostics of diseases in their early stages [5-9]. Nowadays, we can divide nanomedicinal approaches into two sections: i) currently in used and ii) real future perspectives. In the approaches with real future perspectives, we may see therapeutic abilities of nanorobots in microsurgery and in treatment of various types of diseases, such as coronary thrombosis or malignant tumors. We can expect that medicinal nanorobots with size up to 1,000 nm can be injected into human body (some milliards of nanorobots correspond to one milliliter). There they can help to immune system, participate in processes of metabolism, perform repairing operations, eventually cluster together into higher structures and form more complicated and effective repairing and protective systems. Bio-implants in the field of skin regeneration (special polymers, silver nanoparticles) are very important nanomedicinal group, which is currently used.
Nanodelivering of a drug belongs to other nanomedicinal group which gained the great attention of researchers. The development of each new drug has basically two main aspects – maximal effectiveness against the existing disease and as minimal side effects (effect on non-target tissue) as possible (Fig. 2). However, some difficulties connected with the drug delivery may occur, such as troublesome solubility and biological availability, short time of circulation in blood channels, inconvenient biodistribution to the target organ. Nanoparticle-mediated targeted delivery of drugs might significantly reduce the dosage of the drugs, optimized drug loading and release properties, ,increase its specificity and bioavailability including shelf-life and reduce the toxicity [10-12]. These advantages of nanoparticles compared to convenient small molecules for chemotherapy make them promising candidate to combat cancer drug resistance [13]. From the point of view of diseases, in cancer therapy, targeted delivery in a localized way is one of the key challenges. Tumor targeting with nanoparticles can be realized through passive and active way [12, 14-16]. The particles can be modified with various types of materials including biomolecules (Fig. 3). Using various organizations of atoms, resulting properties of particular material, such as elasticity, plasticity, strength, or conductivity can be modified.
The first mentioned improvement in cancer therapy is based on the enhanced permeability and retention (EPR) effect of the vasculature surrounding tumors. The active way relies on ligand-directed binding of nanoparticles to receptors expressed by tumor cells [17]. These ligands comprise antibodies, peptides, nucleic acid aptamers, carbohydrates and small molecules [11]. The key features of anticancer nanoparticles are mainly nanoparticles size, surface properties (e.g. hydrophobicity) and targeting ligands (Fig. 3). Generally, 200 nm is considered as upper limit for nanoparticles size, while the minimal diameter should be about 10 nm. Certainly, nanoparticles properties requirements also depend on tumor characteristics including cancer type, stage of disease, site in the body and host species, which is summarized in review of Adiseshaiah et al. [18].
Nanoparticles designed for tumor targeted therapies consist of various components, in most cases from nanocarrier and an active agent (drug) [19]. Drug-carrier nanoparticles are considered as submicroscopic colloidal systems that may act as drug vehicles, either as nanospheres (matrix system in which the drug is dispersed) or nanocapsules (reservoirs in which the drug is confined in hydrophobic or hydrophilic core surrounded by a single polymeric membrane) [20]. Nanoparticle carriers are mostly composed of iron oxides, gold, biodegradable polymers, dendrimers, lipid based carriers such as liposomes and micelles, viruses (viral nanoparticles) and even organometallic compound [11, 21, 22]. A detailed review about nanocariers was recently published by Peer et al [23]. The drug encapsulation in nanocarrier provides better biocompatibility and hence its potential use in clinical oncology. Several such engineered drugs are already in clinical practice, including liposomal doxorubicin and albumin conjugate paclitaxel [24]. However, the potential success of these particles in the clinic relies on consideration of above mentioned important parameters, but most importantly, minimum toxicity of the carrier itself [25]. Concerning the nanoparticles shape, following nanostructures are frequently cited in literature: nanoshells, nanorods, nanocages, nanocubes or nanotubes.
Podpořeno projekty: Ceitec
Plakáty k výzkumným směrům
Dokumenty pro VaV aktivity
Výzkumný záměr
Hodnocení výzkumných aktivit
Archív

 | Zemědělská 1/1665 613 00 Brno Budova D | Tel.: +420 545 133 350 Fax.: +420 545 212 044 |  |
 |





