

Vědět a znát, to mnohý by chtěl, ale učit se nechce.
Walther Von Der Vegelweide
Výzkum
Structural changes of DNA after interaction with zinc ions as a possible regulatory mechanism in DNA replication
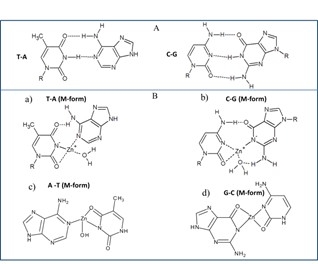
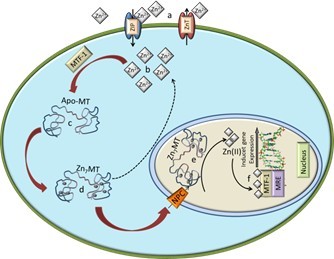 Zinc is a biogenic element that plays an important role in all living organisms. In addition to the fact that zinc is involved in the synthesis of proteins and DNA [1], it has been shown to be essential to stabilize the structure and thus the function of a large number of biomolecules including enzymes. It has been established that zinc is involved in more than 300 enzymatic reactions [2, 3]. It occurs in all tissues and fluids in relatively high concentrations. Almost 85 % of total zinc occurs in muscle and bone tissues, 11% in the skin and the liver and the rest of zinc in other tissues. The average amount of zinc in a body of an adult is about 1.4–2.3 g [4, 5]. Zinc is, after iron, the second most widely used transition metal in living organisms [6]. Zinc ions are transported into cells via zinc transporters, especially ZiP1 [9]. Zinc is found intracellulary in the eukaryotic organisms; 40% of total zinc is located in the nucleus, 50% in the cytoplasm, organelles, and specialized vesicles called zincosomes and the rest o zinc is bound in the cell membrane [7]. The concentration of zinc is relatively high in nucleus. It has been shown that zinc is involved in the processes of gene expression and the maintenance of gene stability by different ways. It stabilizes the structure of chromatin and thus affects the replication of DNA; in addition, zinc regulates transcription of RNA through the regulation of an activity of transcription factors and some enzymes, such as RNA and DNA polymerases [8]. The structural role of zinc ions is accentuated in the stabilization of zinc finger motif [10]. It is an amino acid sequence linked to zinc ion to form a secondary structure in the shape of a finger [10]. Proteins with this motif have high affinity to DNA, so, zinc finger directly mediates an interaction with DNA in the major groove [11]. Zinc is the main component of the zinc finger proteins, which represent the largest and the most diverse superfamily of nucleic acid-binding proteins and which play important roles in the regulation of transcription in the cellular metabolic network [12, 13]. Increased amount of zinc, respectively zinc ions, causes an increased expression of apo-metallothionein (apo-MT) via metal-regulatory transcription factor 1 (MTF-1). Apo-MT is able to bind seven atoms of Zn(II), which can probably be transported in this complex through the nuclear pores (NPC) into the nucleus (Fig. 1). MTF-1 is reversibly bound to target DNA, so, the binding depends on changes in an availability of free Zn(II) in the cytoplasm [14, 15]. The binding of MTF-1 to the metal regulatory element, MRE, requires activation by zinc ions. In conclusion, zinc ions can enter the nucleus to activate expression of MT genes from a dietary source. Once MTF-1 senses free Zn(II), it adopts a reversible DNA-binding conformation. This allosteric change causes an exposure of zinc fingers, and the MTF-1 can move to the nucleus and activate gene expression by an association with the gene promoters that carry MREs [16]. Study by Jiang et al. has confirmed that the mediation of MT gene expression starts by a translocation of MTF-1 protein from the cytoplasm to a nucleus and bind MREs in target genes [16]. However, whether metal ions can induce the creation of MTF-1 in the cytoplasm and how do other metal ions induce MT via MTF-1 remain to be elucidated [17]. A study of DNA–metal ion complex, known as M-DNA, in which a divalent metal ion is incorporated into the centre of the DNA duplex, has been performed [18]. This metal ion complex was firstly named as M-DNA in 1993 [19]. Since that time, many studies that describe the creation, character and application of this complex, has been published [20-33]. The biological role of the interaction of Zn(II) with proteins is relatively well known and exploited. The question is what role has M-DNA complex in living organisms and how does it affect the regulation of transcription and replication of DNA, or what relation has M-DNA in a processes of cancerogenesis. The proposed structure of M-DNA that is based on the NMR, CD, and molecular modelling studies consists of GC and AT base pairs, in which the imino-proton of G and T is replaced by Zn(II), which results in an atomically thick “wire” of zinc ions sheathed by a DNA helix [20]. Zinc, respectively zinc ions also bind to phosphate groups of DNA and are able to influence (”destroy”) the basic structure of B conformation of DNA [34]. The preferred site for the coordination of the metal ion to the DNA is the N7 position of guanines [35]. Conformational change of ds or ssDNA after interaction with zinc ions may be used in the regulation of transcription [36]. It has been found that the TGGGA sequence of the Xenopus 5S-RNA gene, which is essential for binding the transcription factor TFIIIA, has the highest affinity for zinc ions [35].Various techniques have been applied to investigate the interactions of heavy metal ions with DNA. These techniques include FTIR difference spectroscopy [23], Raman spectroscopy [34], and circular dichroism spectroscopy [37] . However, these techniques have high demands on special equipment and are not commonly available. The aim of this work was to study the interaction of zinc ions with a fragment of DNA by UV/VIS spectrophotometry (changes in absorption signals and melting temperatures in denaturation) and gel electrophoresis (changes in the mobility of DNA fragments) after purification on a semi-permeable membrane.
Zinc is a biogenic element that plays an important role in all living organisms. In addition to the fact that zinc is involved in the synthesis of proteins and DNA [1], it has been shown to be essential to stabilize the structure and thus the function of a large number of biomolecules including enzymes. It has been established that zinc is involved in more than 300 enzymatic reactions [2, 3]. It occurs in all tissues and fluids in relatively high concentrations. Almost 85 % of total zinc occurs in muscle and bone tissues, 11% in the skin and the liver and the rest of zinc in other tissues. The average amount of zinc in a body of an adult is about 1.4–2.3 g [4, 5]. Zinc is, after iron, the second most widely used transition metal in living organisms [6]. Zinc ions are transported into cells via zinc transporters, especially ZiP1 [9]. Zinc is found intracellulary in the eukaryotic organisms; 40% of total zinc is located in the nucleus, 50% in the cytoplasm, organelles, and specialized vesicles called zincosomes and the rest o zinc is bound in the cell membrane [7]. The concentration of zinc is relatively high in nucleus. It has been shown that zinc is involved in the processes of gene expression and the maintenance of gene stability by different ways. It stabilizes the structure of chromatin and thus affects the replication of DNA; in addition, zinc regulates transcription of RNA through the regulation of an activity of transcription factors and some enzymes, such as RNA and DNA polymerases [8]. The structural role of zinc ions is accentuated in the stabilization of zinc finger motif [10]. It is an amino acid sequence linked to zinc ion to form a secondary structure in the shape of a finger [10]. Proteins with this motif have high affinity to DNA, so, zinc finger directly mediates an interaction with DNA in the major groove [11]. Zinc is the main component of the zinc finger proteins, which represent the largest and the most diverse superfamily of nucleic acid-binding proteins and which play important roles in the regulation of transcription in the cellular metabolic network [12, 13]. Increased amount of zinc, respectively zinc ions, causes an increased expression of apo-metallothionein (apo-MT) via metal-regulatory transcription factor 1 (MTF-1). Apo-MT is able to bind seven atoms of Zn(II), which can probably be transported in this complex through the nuclear pores (NPC) into the nucleus (Fig. 1). MTF-1 is reversibly bound to target DNA, so, the binding depends on changes in an availability of free Zn(II) in the cytoplasm [14, 15]. The binding of MTF-1 to the metal regulatory element, MRE, requires activation by zinc ions. In conclusion, zinc ions can enter the nucleus to activate expression of MT genes from a dietary source. Once MTF-1 senses free Zn(II), it adopts a reversible DNA-binding conformation. This allosteric change causes an exposure of zinc fingers, and the MTF-1 can move to the nucleus and activate gene expression by an association with the gene promoters that carry MREs [16]. Study by Jiang et al. has confirmed that the mediation of MT gene expression starts by a translocation of MTF-1 protein from the cytoplasm to a nucleus and bind MREs in target genes [16]. However, whether metal ions can induce the creation of MTF-1 in the cytoplasm and how do other metal ions induce MT via MTF-1 remain to be elucidated [17]. A study of DNA–metal ion complex, known as M-DNA, in which a divalent metal ion is incorporated into the centre of the DNA duplex, has been performed [18]. This metal ion complex was firstly named as M-DNA in 1993 [19]. Since that time, many studies that describe the creation, character and application of this complex, has been published [20-33]. The biological role of the interaction of Zn(II) with proteins is relatively well known and exploited. The question is what role has M-DNA complex in living organisms and how does it affect the regulation of transcription and replication of DNA, or what relation has M-DNA in a processes of cancerogenesis. The proposed structure of M-DNA that is based on the NMR, CD, and molecular modelling studies consists of GC and AT base pairs, in which the imino-proton of G and T is replaced by Zn(II), which results in an atomically thick “wire” of zinc ions sheathed by a DNA helix [20]. Zinc, respectively zinc ions also bind to phosphate groups of DNA and are able to influence (”destroy”) the basic structure of B conformation of DNA [34]. The preferred site for the coordination of the metal ion to the DNA is the N7 position of guanines [35]. Conformational change of ds or ssDNA after interaction with zinc ions may be used in the regulation of transcription [36]. It has been found that the TGGGA sequence of the Xenopus 5S-RNA gene, which is essential for binding the transcription factor TFIIIA, has the highest affinity for zinc ions [35].Various techniques have been applied to investigate the interactions of heavy metal ions with DNA. These techniques include FTIR difference spectroscopy [23], Raman spectroscopy [34], and circular dichroism spectroscopy [37] . However, these techniques have high demands on special equipment and are not commonly available. The aim of this work was to study the interaction of zinc ions with a fragment of DNA by UV/VIS spectrophotometry (changes in absorption signals and melting temperatures in denaturation) and gel electrophoresis (changes in the mobility of DNA fragments) after purification on a semi-permeable membrane.
Podpořeno projekty: NANOBIOTECELL
Apoferritin Modified Magnetic Particles as Doxorubicin Carriers for Anticancer Drug Delivery
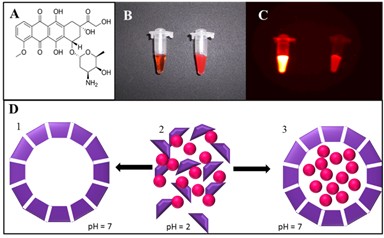 Magnetic particles can be of the size of several nanometers to several micrometers consisted mainly of iron, nickel, cobalt and gadolinium [1-6]. Magnetic nanoparticles with appropriate surface coatings can be used for various biomedical purposes, such as drug delivery, hyperthermia, tissue repairing, cell and tissue targeting, transfection and magnetic resonance imaging [2,7-9]. Surface functionalization allows us to use nanoparticles as probes for molecular imaging [10]. The material employed for surface coating of the magnetic particles must be nontoxic and biocompatible and has to enable us a targeted delivery with localization in a required area [2,11]. Nanoparticles have a large surface area and provide a large number of functional groups for cross-linking to tumor-targeting ligands such as monoclonal antibodies, peptides, or small molecules for diagnostic imaging or delivery of therapeutic agents [12,13]. The linkage of the drug with magnetic nanoparticle has to be stable to prevent drug release during its transport [14]. There can be used varied surface modifications for the biomedical applications [11,15-17]. Polymers, poly(ethylene glycol) (PEG), N-(2-hydroxypropyl)methacrylamide (HPMA), and poly(lactide-co-glycolide) (PLGA) copolymers have been successfully utilized in clinical research [14]. Surface modifications can be also carried out using tetraethoxysilane (TEOS), triethoxysilane (TES) and 3-aminopropyltrimethoxysilane (APTMS) [6]. The transport to the vessel wall is essential for localizing therapy [18].
Magnetically driven superparamagnetic iron oxide could be used as an emerging therapeutic delivery system [19]. Anticancer drugs reversibly bound to magnetic fluids (ferrofluids) could be concentrated in tumors by magnetic fields that are arranged at the tumor surface outside of the organism [20]. Moreover, delivery by magnetic particles can be coupled to specialized nanocarriers such as lipid- [21] and/or protein-based carriers [22,23] enabling selective release of the drug in the site of the action. Such release may be performed by various mechanisms including photo- [24] or thermoiniciated [25] or pH triggered release [23,26]. In this study, magnetic particle-based targeted, apoferritin mediated and pH triggered transport of doxorubicin (DOX) was studied using capillary electrophoresis with laser-induced fluorescence detection.
.
Magnetic particles can be of the size of several nanometers to several micrometers consisted mainly of iron, nickel, cobalt and gadolinium [1-6]. Magnetic nanoparticles with appropriate surface coatings can be used for various biomedical purposes, such as drug delivery, hyperthermia, tissue repairing, cell and tissue targeting, transfection and magnetic resonance imaging [2,7-9]. Surface functionalization allows us to use nanoparticles as probes for molecular imaging [10]. The material employed for surface coating of the magnetic particles must be nontoxic and biocompatible and has to enable us a targeted delivery with localization in a required area [2,11]. Nanoparticles have a large surface area and provide a large number of functional groups for cross-linking to tumor-targeting ligands such as monoclonal antibodies, peptides, or small molecules for diagnostic imaging or delivery of therapeutic agents [12,13]. The linkage of the drug with magnetic nanoparticle has to be stable to prevent drug release during its transport [14]. There can be used varied surface modifications for the biomedical applications [11,15-17]. Polymers, poly(ethylene glycol) (PEG), N-(2-hydroxypropyl)methacrylamide (HPMA), and poly(lactide-co-glycolide) (PLGA) copolymers have been successfully utilized in clinical research [14]. Surface modifications can be also carried out using tetraethoxysilane (TEOS), triethoxysilane (TES) and 3-aminopropyltrimethoxysilane (APTMS) [6]. The transport to the vessel wall is essential for localizing therapy [18].
Magnetically driven superparamagnetic iron oxide could be used as an emerging therapeutic delivery system [19]. Anticancer drugs reversibly bound to magnetic fluids (ferrofluids) could be concentrated in tumors by magnetic fields that are arranged at the tumor surface outside of the organism [20]. Moreover, delivery by magnetic particles can be coupled to specialized nanocarriers such as lipid- [21] and/or protein-based carriers [22,23] enabling selective release of the drug in the site of the action. Such release may be performed by various mechanisms including photo- [24] or thermoiniciated [25] or pH triggered release [23,26]. In this study, magnetic particle-based targeted, apoferritin mediated and pH triggered transport of doxorubicin (DOX) was studied using capillary electrophoresis with laser-induced fluorescence detection.
.
Podpořeno projekty: CYTORES
Plakáty k výzkumným směrům
Dokumenty pro VaV aktivity
Výzkumný záměr
Hodnocení výzkumných aktivit
Archív

 | Zemědělská 1/1665 613 00 Brno Budova D | Tel.: +420 545 133 350 Fax.: +420 545 212 044 |  |
 |





