

Tajemství vládnutí spočívá totiž v tom, jak spojit víru ve vlastní neomylnost se schopností učit se z minulých chyb.
George Orwell
Výzkum
Electrochemical Study of Ellipticine Interaction with Single and Double Stranded Oligonucleotides
 Ellipticine (5,11-dimethyl-6H-pyrido[4,3-b]carbazole) and its 9-methoxyderivate are natural substances isolated from some plant species of an Apocynaceae family such as Ochrosia borbonica and Excavatia coccinea [1-6]. These chemicals have only limited haematological toxicity, fairly limited toxic side effects but strong antitumor effects from clinical point of view [1-6], Based on these reasons, the promising results have been shown in the treatment of several tumours particularly such as myeloblastic leukaemia and bone metastases of breast-cancer with this drug [1-6]. Ellipticine within the concentration range from 10-10 to 10- 6 M induces growth arrest and cells death in breast adenocarcinoma [7-9], leukaemia [10], colon cancer [11], neuroblastoma [12], glioblastoma [13, 14], lung carcinoma [15] and hepatocellular carcinoma [16] cells. Due to the high efficiency (antitumour activity) of ellipticine and its derivatives against various types of cancer, the interest on this drug has been increasing. New directions for development and preparation of targeted drugs based on ellipticine are still explored. Furthermore, ellipticine also exhibited anti-HIV activity [17].
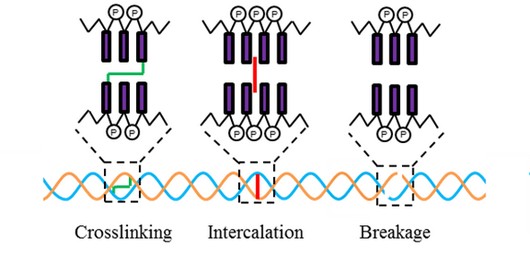
Cytotoxic and mutagenic effects of ellipticine are attributed mainly to two mechanisms, intercalation into the structure of DNA double helix [18] and inhibition of topoisomerase II [19]. The intercalation effect is the first reported mechanism. Ellipticine itself is not too strong intercalator, but more effective from this point of view are its hydroxyderivates. The 9-hydroxyellipticine shows the best affinity to the guanine - cytosine (GC) [for a review see 1-6]. Several studies showed that the planar (hetero)-aromatic structure intercalated between pairs of bases of double-stranded DNA usually leads to its stabilization, strengthening of conformations, elongation and partially untangle [1-6]. These changes usually lead to functional inhibition of the numerous enzyme processes acting on DNA, such as transcription and replication [3, 6].
Ellipticine (5,11-dimethyl-6H-pyrido[4,3-b]carbazole) and its 9-methoxyderivate are natural substances isolated from some plant species of an Apocynaceae family such as Ochrosia borbonica and Excavatia coccinea [1-6]. These chemicals have only limited haematological toxicity, fairly limited toxic side effects but strong antitumor effects from clinical point of view [1-6], Based on these reasons, the promising results have been shown in the treatment of several tumours particularly such as myeloblastic leukaemia and bone metastases of breast-cancer with this drug [1-6]. Ellipticine within the concentration range from 10-10 to 10- 6 M induces growth arrest and cells death in breast adenocarcinoma [7-9], leukaemia [10], colon cancer [11], neuroblastoma [12], glioblastoma [13, 14], lung carcinoma [15] and hepatocellular carcinoma [16] cells. Due to the high efficiency (antitumour activity) of ellipticine and its derivatives against various types of cancer, the interest on this drug has been increasing. New directions for development and preparation of targeted drugs based on ellipticine are still explored. Furthermore, ellipticine also exhibited anti-HIV activity [17].
Cytotoxic and mutagenic effects of ellipticine are attributed mainly to two mechanisms, intercalation into the structure of DNA double helix [18] and inhibition of topoisomerase II [19]. The intercalation effect is the first reported mechanism. Ellipticine itself is not too strong intercalator, but more effective from this point of view are its hydroxyderivates. The 9-hydroxyellipticine shows the best affinity to the guanine - cytosine (GC) [for a review see 1-6]. Several studies showed that the planar (hetero)-aromatic structure intercalated between pairs of bases of double-stranded DNA usually leads to its stabilization, strengthening of conformations, elongation and partially untangle [1-6]. These changes usually lead to functional inhibition of the numerous enzyme processes acting on DNA, such as transcription and replication [3, 6].
The other mechanisms of the ellipticine cytotoxic effect mediated by DNA damage are that ellipticine induces DNA strand breaks connected with topoisomerase II inhibition [20, 21] and it also forms covalent DNA adducts after its activation with peroxidase and cytochrome P450 enzymes [3-6, 22]. The induction of DNA strand breaks was described for the first time in DNA from L1210 cells [23]. The unsuccessful reparation of DNA faults leads to apoptosis of cancer cells. The major study about ellipticine-topoisomerase activity identified topoisomerase II as the primary cellular target for this drug [24]. Cytochrome P450 enzymes metabolize ellipticine to five metabolites: 13-hydroxyellipticine, 12-hydroxyellipticine and N2-oxide of ellipticine, 7-hydroxyellipticine and 9-hydroxyellipticine [5, 6, 25]. 12-Hydroxyellipticine, 13-hydroxyellipticine and the ellipticine N2-oxide (generating spontaneously 12-hydroxyellipticine) are responsible for formation of ellipticine-12/13-ylium that react with nucleophilic centres of the deoxyguanosine residues in DNA, generating two DNA adducts [3, 5, 6, 22, 25, 26]. In the case of peroxidases, ellipticine is oxidized to radicals providing ellipticine dimer (major product) and, in a minority, the N2-oxide of ellipticine [22, 27]. Using the 32P-post-labeling method and [3H]-labelled ellipticine, Stiborova et al. showed that ellipticine binds covalently to DNA after its activation both by peroxidases [22, 27] and cytochromes P450 [3-6, 25]. Nowadays electrochemistry is considered as one of the most sensitive method for detection of DNA [3, 24]. Moreover, these methods can be used for detection of DNA adducts through determination of DNA peaks (mostly cytosine-adenine (CA) peak and guanine peak (G) [28]) and peak connected with drugs. CA peak can be determined by differential pulse voltammetry (DPV) and G peak by square wave voltammetry (SWV). The aim of the present study was to characterize ellipticine and its interaction with single (ssODN) and double (dsODN) strand oligonucleotides electrochemically by adsorptive transfer stripping technique (AdTS) at hanging mercury drop electrode (HMDE).
Plakáty k výzkumným směrům
Dokumenty pro VaV aktivity
Výzkumný záměr
Hodnocení výzkumných aktivit
Archív

 | Zemědělská 1/1665 613 00 Brno Budova D | Tel.: +420 545 133 350 Fax.: +420 545 212 044 |  |
 |




